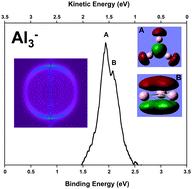
Aluminium bridges the gap between monovalent and multivalent clusters, as it is monovalent as a single atom, but becomes trivalent with hybridized orbitals at larger cluster sizes. The exact cluster size at which this hybridisation occurs is still the matter of intense debate, with little coherence amongst the results of a large number of studies, both experimental and theoretical.
Melko and Castleman attempt to resolve this problem by conducting both a theoretical study and an experimental study using the angular distribution information obtained from photoelectron imaging. They then developed a calibration curve that allowed them to quantitatively compare their results, which were rather surprising. They suggest that the orbital hybridisation that indicates bulk behaviour begins to appear at cluster sizes as small as Al3–, which is considerably smaller than previously thought. The extent of the hybridisation then appears to oscillate as successive atoms are added to the cluster up to at least Al6–, suggesting the existence of a transition period between the monovalent and trivalent states, rather than a discrete threshold.
by Victoria Wilton
Read the full details of this fascinating PCCP article:
Photoelectron imaging of small aluminum clusters: quantifying s–p hybridization
Joshua J. Melko and A. W. Castleman
DOI: 10.1039/C3CP43158D
If you enjoyed this paper you may also be interested in the Nanoscale themed issue on Metallic clusters – please do take a look.










