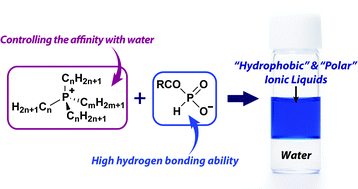
The authors found that tetra-n-octylphosphonium paired with ethylphosphonate or n-butylphosphonate were able to form a stable seperate phase after mixing with water while also showing hydrogen bonding characteristics.
This family of ILs could potentially be used in environmentally friendly separation processes.
Read this exciting PCCP article in full:
Hydrophobic and polar ionic liquids
Yukinobu Fukaya and Hiroyuki Ohno
DOI: 10.1039/C3CP44214D
If you liked this you may also enjoy our recent PCCP themed issue on Interfaces of Ionic Liquids Guest Edited by Professor Frank Endres.










