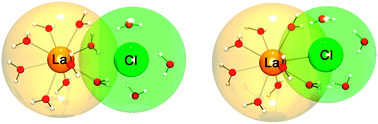
Researchers in the group of Professor Martina Havenith at the Ruhr-University Bochum have shown that terahertz spectroscopy can be used, employing meticulous physical chemical considerations, to open a window into the solvation of ions in solution. In this paper they join the discussion regarding the solvation of trivalent lanthanide ions in water, by studying lanthanum chloride and bromide at different concentrations. They determine association constants for the ion pairs and investigate the nature of the lanthanide solvation—adding the experimental support to the proponents of outer-sphere interaction between anions and trivalent lanthanide ions in solution.
Sharma and co-workers investigate the ion pairing of lanthanum halides from a true physical chemical approach. A puritan approach that to me, make this paper a pivotal example of what a PCCP paper should be. Is should not necessarily following the most recent trend, but answer or address an important question. Here, the hydration of lanthanide ions. We do not know the structure of solvation lanthanide ions, even the stoichiometry is unknown. Except for an assumption that lanthanum has nine water molecules in the ligand sphere forming a symmetrical tricapped trigonal prism, the paper attacks the question of lanthanide solvation from a refreshingly new angle.
The image attached to this post shows how well terahertz spectroscopy can describe a solution of ions. Remembering that this image is generated from several layers of a priori information, such as the behavior of neat water, simple electrolytes, solutions of alkali halides etc. I strongly recommend that you read the paper is you are interested in the rare earths, electrolytes or specific ion effects.
by Dr Thomas Just Sørensen
Read this exciting PCCP article today:
From solvated ions to ion-pairing: a THz study of lanthanum(III) hydration
Vinay Sharma, Fabian Böhm, Michael Seitz, Gerhard Schwaab and Martina Havenith
Phys. Chem. Chem. Phys. 2013, 15, 8383-8391
DOI: 10.1039/C3CP50865J