Hydrogen is the only element in the periodic table that is not truly part of a group, although it is often nominally assigned to group 1. All chemists are familiar with the concept of the hydrogen bond, but how many think of the halogen bond in the same light? How many are even aware of the halogen bond as a special entity, less still that the two interactions are to all intents and purposes the same thing?
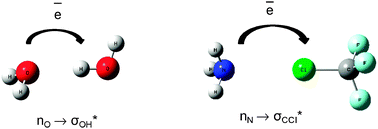
In his recent paper, Grabowski uses theoretical techniques to show that both interactions are ruled by the same electrostatic mechanism. He also provides an excellent summary and comparison of the information currently known about the two interactions that indicates a clear progression in some bonding properties from hydrogen through to the heavy halogens.
He describes how the atomic volume of the halogen decreases as the positive charge on it increases, and that this effect is magnified by shortening the internuclear distance. This information accompanies the observation that the strength of the Lewis acid-base interaction increases with the increasing atomic number of the halogen involved, although with some exceptions, hydrogen bonds are generally stronger still.
This discovery clearly has important implications for our understanding of non-covalent molecular interactions, and our understanding of how best to classify hydrogen based on its bonding properties.
by Victoria Wilton
Read this HOT PCCP article today:
Hydrogen and halogen bonds are ruled by the same mechanisms
Sławomir J. Grabowski
DOI: 10.1039/C3CP50537E