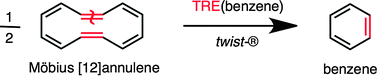Remi Chauvin and Christine Lepetit give the topological resonance energy (TRE) acyclic reference of any π-cyclic molecule, for 36 years merely defined by an abstract matching polynomial, a real chemical structure: the Mobius-twisted head-to-tail metathesis cyclo-dimer of the parent ring.
The aromaticity of a cyclically conjugated molecular system can be qualitatively defined as its tendency to resist the loss of its cyclic character under constraint or relief of external perturbations, or fundamentally quantified as the difference between the energy of the cyclic system and the energy of some acyclic reference. This acyclic reference is rigorously defined as the abstract reference of the TRE, but might not exist as a chemical species. In their recent PCCP paper, Chauvin and Lepitit report the long-sought chemical nature of TRE, and of topological aromaticity.
Read the details in this HOT article today:
The fundamental chemical equation of aromaticity
Remi Chauvin and Christine Lepetit
DOI: 10.1039/C2CP44075J