Hernandez and Schmidt investigate the role of anisotropic water-carbon interactions on water in low-diameter carbon nanotubes (CNTs) in their recent PCCP paper, and demonstrate the importance of the anisotropy. Extensive simulations provide a comprehensive picture of the effects of the interaction anisotropy on the structure and dynamics of water confined in narrow, single-walled CNTs.
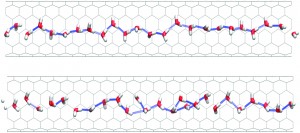
Carbon based materials are generally accepted as being hydrophobic, but the calculated water-graphene interaction is found to be non-negligible and, moreover, orientation-dependant. The authors firstly calibrated new parameters for a Lennard-Jones potential, emphasising the anisotropy in the carbon-water interaction. They then performed molecular dynamics studies, using these parameters, of water inside various CNTs to examine properties such as structure, Lindemann index, mean square displacements and H-bonding patterns.
In contrast to previous simulations employing spherical interaction models, they found that the water molecules tend to form denser clusters displaying liquid-like behaviour, allowing for self-diffusion along the CNT axis.
Structures and hydrogen-bonding networks of water molecules inside CNTs can be radically different to those found in bulk water, due to the fact that the water molecules are confined in tubes with diameters not much larger than their own size. A central issue in many applications of CNTs is the possibility of conveying or storing fluids, hence the importance of understanding these systems.
Read about these fascinating results in this article:
Anisotropy of the Water-Carbon Interaction: Molecular Simulations of Water in Low-Diameter Carbon Nano-Tubes
Burkhard Schmidt
DOI: 10.1039/C3CP44278K










