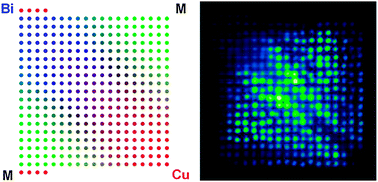
A new dispenser and scanner system has been developed that creates and screens arrays of different metal oxides, assessing there suitability for photocatalytic water-splitting reactions. The technique operates in a combinatorial fashion and has been used to screen over 3000 unique Bi:M:Cu atomic ratios, where M represents one of 22 post-transition metals.
Of the 22 metals tested, 10 were found to have a M-Cu oxide with higher photochemical activity than CuO, while 10 had a Bi-M-Cu oxide with more activity than CuBi2O4. The best performing combination was BiAgCu oxide with the ratio 22:3:11, which produced a photocurrent four times that of CuBi2O4. The material was capable of evolving hydrogen from neutral electrolyte solutions under illumination at 0.6V vs RHE when platinum was added as an electrocatalyst.
Read the full details of this fascinating PCCP article:
Screening of transition and post-transition metals to incorporate into copper oxide and copper bismuth oxide for photoelectrochemical hydrogen evolution
Sean P. Berglund, Heung Chan Lee, Paul D. Núñez, Allen J. Bard and C. Buddie Mullins
DOI: 10.1039/C3CP50540E