 Heterogenic catalysis and the oxidation of carbon monoxide to carbon dioxide would generally not qualify as interesting in my book. But this piece of work from the group of Professor Ib Chorkendorff fascinates me, as does all phenomena where an process appears to be oscillating without external stimulus to do so.
Heterogenic catalysis and the oxidation of carbon monoxide to carbon dioxide would generally not qualify as interesting in my book. But this piece of work from the group of Professor Ib Chorkendorff fascinates me, as does all phenomena where an process appears to be oscillating without external stimulus to do so.
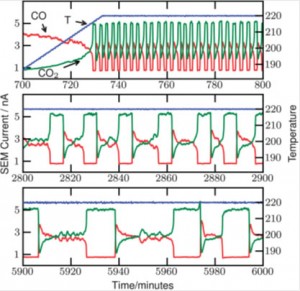
A delicate balance of chemical equilibria is found to be responsible for the oscillations observed by Jensen and co-workers, and in the paper they are able to account for their findings.
They found the oscillations as they were studying the oxidation of CO to CO2 in an O2 rich atmosphere. The oxidation is catalysed by platinum; in this work they use Pt-particles of a similar size on a silicon oxide surface. This fact is found to be decisive, as the oscillations only occur if particles are used.
For a full explanation I must refer to:
Self-sustained carbon monoxide oxidation oscillations on size-selected platinum nanoparticles at atmospheric pressure
Robert Jensen, Thomas Andersen, Anders Nierhoff, Thomas Pedersen, Ole Hansen, Søren Dahl and Ib Chorkendorff
DOI: 10.1039/C2CP43684A
by Dr Thomas Just Sørensen










