Grant McIntosh from the University of Auckland, New Zealand, has shone light in the interesting problem of silicate oligomer formation kinetics. For the first time, an ab initio study has been conducted that considers all possible oligomerization reactions available to silicic acid in basic solution, up to and including tetramers. An understanding of the nucleation and growth of silica colloids is important in the comprehension of sol-gel processes and geothermal fluids.
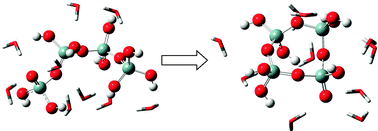 Including explicit water molecules in the calculations showed that these greatly affect the stability of intermediates and transition states, suggesting that some of the known pathways are more facile than previously predicted. Previously neglected bimolecular growth pathways were found to be energetically feasible, and so could significantly impact on the intial stages of silica nucleation.
Including explicit water molecules in the calculations showed that these greatly affect the stability of intermediates and transition states, suggesting that some of the known pathways are more facile than previously predicted. Previously neglected bimolecular growth pathways were found to be energetically feasible, and so could significantly impact on the intial stages of silica nucleation.
Silicates are able to adopt a vast array of linear, cyclic, and branched structures, which makes it tough to experimentally monitor and identify product oligomers of silicate systems. This challenge is complicated further by the sensitivity toward temperature, ionic strength and pH. McIntosh remarks that a full picture of silicate growth will require time- and concentration dependent modelling techniques.
Read McIntosh’s article today:
Theoretical investigations into the nucleation of silica growth in basic solution part I – ab Initio studies of the formation of trimers and tetramers
Grant J. McIntosh
DOI: 10.1039/C3CP43399D










