It is now possible to accurately predict the density, refractive index and molar polarisability of any imidazolium-based ionic liquid thanks to the recent work of Schröder et al.
Given the availability of at least one million simple ionic liquids, predicting which ionic liquid is best suited to a given application is a significant task. Molar polarisability is a key factor in describing solvation effects and, in principle, can be determined by various quantum-mechanical methods. However, these methods are 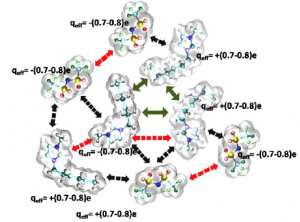 time-consuming and can only be applied to a small subset of ionic liquids.
time-consuming and can only be applied to a small subset of ionic liquids.
Schroder et al. used a Designed Regression Analysis to deconstruct the molar polarisability and molar volume into atomic contributions in this recent PCCP article. They used their approach to explore how the refractive indices of various imidazolium-based ionic liquids were influenced by the length of the alkyl chains.
Read the full PCCP article today:
Polarisabilities of alkylimidazolium ionic liquids
Christian Schröder, Katharina Bica, Maggel Deetlefs and Kenneth R. Seddon
DOI: 10.1039/C3CP43867H