D. Qi, C. K. Chan, and A. C. Rowat*
Department of Integrative Biology and Physiology, University of California, Los Angeles, 610 Charles E. Young Drive East, Los Angeles, CA 90095, USA
* e-mail: rowat[at]ucla.edu
Why is this useful?
A major challenge in microfluidics is interfacing micron-scale flow channels with fluid samples and peripheral equipment of the macroscopic world, such as plate readers and liquid-handling robots. To exchange fluids in and out of the device, inlet and outlet holes are essential. In polydimethylsiloxane (PDMS) microfluidic devices, small <1 mm inlet- and outlet-holes are typically used to interface fluidic samples and micron-scale channels. These holes are typically fabricated by manually excising PDMS slabs to produce holes, for example using a needle or razor-sharp biopsy punch. However, to achieve scale-up of microfluidic devices can require ~10^2 inlets and outlets required for parallelization of multiple samples.
Manually punching holes is tedious due to the following factors: (1) it is often difficult to determine where to punch holes due to the micron-scale dimensions of the channels and transparency of PDMS; (2) the resulting holes do not always have smooth edges, and resultant chunks of PDMS can clog micron-scale channels; (3) biopsy punches used to excise holes become dull after multiple uses and thus frequently need to be replaced; (4) it is difficult to manually punch holes that are vertically straight and have reproducible spatial position; this makes it challenging to achieve the precision required for fabricating devices that robustly interface with existing high throughput equipment, such as liquid-handling robots. Here we present a method to easily and reproducibly fabricate smooth, vertically straight arrays of interfacing holes in PDMS using standard arrays of 96 or 384 pipette tips.
What do I need?
- Common items to prepare PDMS devices: petri dish, master wafer or device mold, PDMS silicone elastomer (base and curing agent), vacuum desiccator, and oven.
- Micropipette tip racks. Depending on microfluidic device designs, users can build their own tip racks; for example, we machined a 96-tip holding rack out of Perspex (polymethylmethacrylate).
- Micropipette tips, e.g., 10-, 20- or 200- μl volume.
- Four 3-inch steel bolts with nuts.
What do I do?
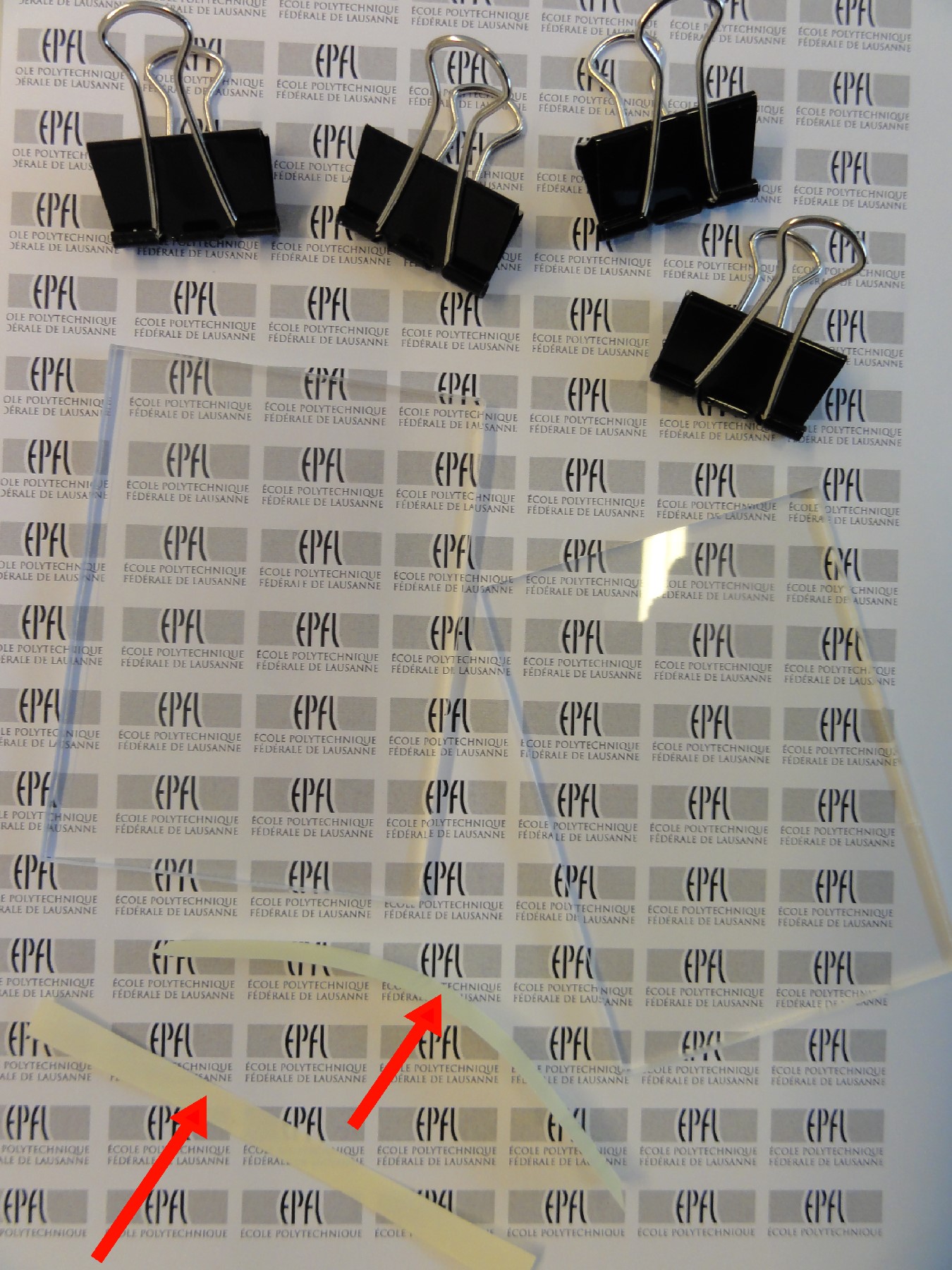
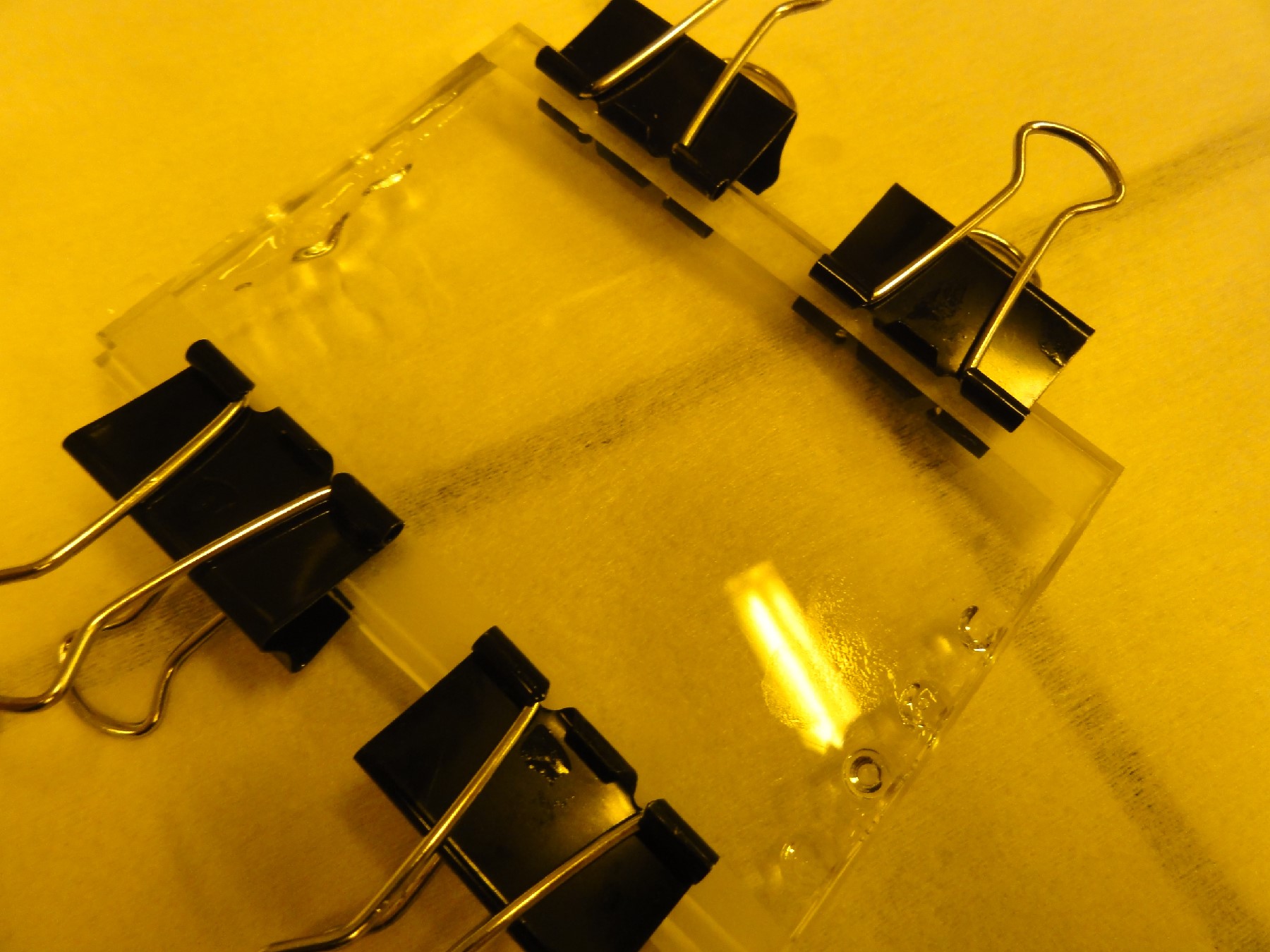
- Assemble tip racks using four steel bolts with nuts (see Figure 1.); the bottom rack is fixed and the top one is adjustable vertically along the bolts.
- Put the petri dish (with the master wafer inside) on the bottom rack.
- Position pipette tips in the top rack.
- Align tips to the inlet- and outlet-hole positions of the desired microfluidic channels; move the top rack down until the tips physically touch the master wafer.
- After the tip alignment, put a flat light weight object, e.g., a Perspex block ~155g, on top of the tips to hold them in position, while also making sure that tips and the master wafer are in physical contact.
- Prepare PDMS silicone elastomer (base-to-curing agent ratio-10:1) and mix well by vigorous stirring.
- Carefully pour the silicone elastomer mixture into the petri dish.
- Put the entire setup in the vacuum desiccator and degas for 20-30 minutes.
- Move the setup into an oven and thermally cure silicone elastomer at 65 degree Celsius for >1 hour.
- After curing, remove the setup from the oven, and let cool to room temperature.
- Using tweezers, gently pull out tips from the thermally cured PDMS.
- Using a razor, cut out the PDMS device with molded channels and holes from the petri dish.
- Bond PDMS to glass coverslips or other substrates, as required for your application.
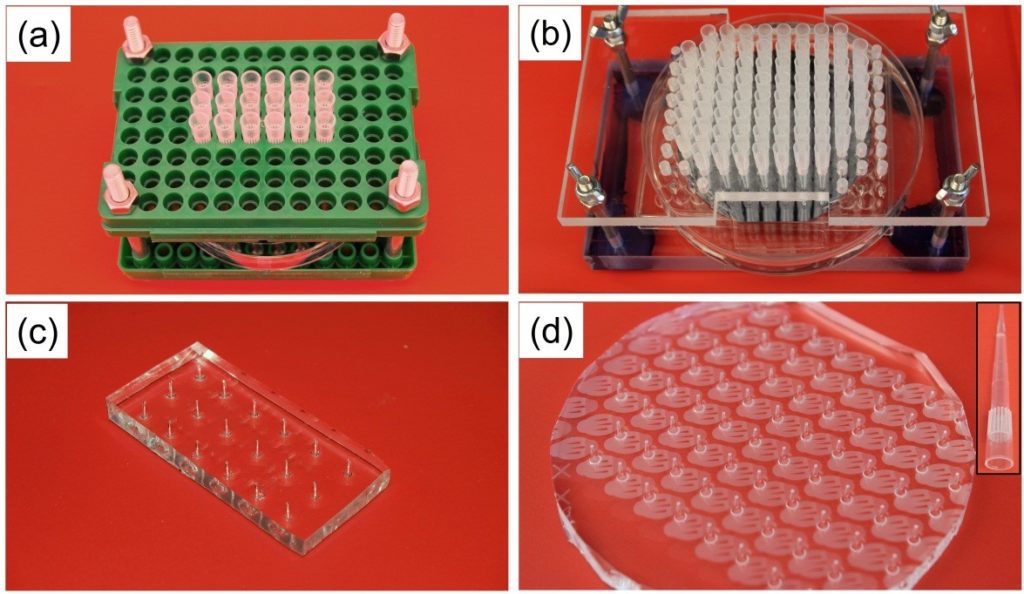
Figure 1. Images of hole-molding setup with (a) standard pipette tip rack, and (b) custom fabricated Perspex holder. Images of the molded holes in PDMS are shown in (c) and (d); inset in (d) shows one micropipette tip with tiny PDMS plug at its tip.
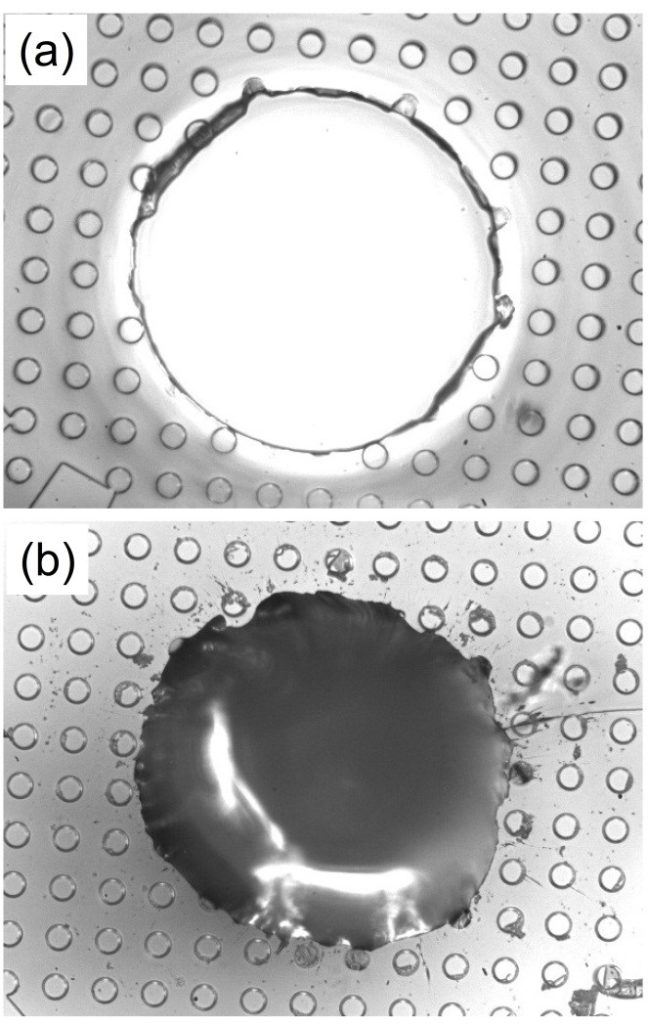
Figure 2. Typical inlet/outlet holes in a PDMS slab (a) molded by our molding method using a 200 μl micropipette tip; and (b) punched by a 0.75mm Harris Uni-Core (TED PELLA) punch. The PMDS slab is patterned with 50 μm cylindered posts.
What else should I know?
- The arrangement of tips can be tuned for specific applications by using a custom-fabricated pipette tip rack to accommodate unique inlet- and outlet-hole arrangements; such as the Perspex rack as shown in Figure 1. (b).
- During molding, a small amount of silicone elastomer is taken up into the micropipette tip ends during degassing in the vacuum chamber (Fig. 1d, inset); this occurs due to the imperfect sealing between the micropipette tips and the master wafer. This tiny plug of PDMS is easily removed from the device together with the pipette tip, resulting in holes that are free of any obstructions.
- When using tubing to interface microfluidic devices with macroscopic equipment, the outside diameter (OD) of the tubing should be larger than the diameter of such mold holes in PDMS to avoid leakage. We have successfully used both 20- and 200- μl micropipette tips to mold holes, which are ~0.7 mm in diameter at the end; 10 µL pipette tips have similar diameter. Both 1⁄32″ and 1⁄16″ OD tubing (VICI Valco Instruments) interfaces well with holes of these dimensions.
- By our proposed molding method, the size of the holes closely follows the outer diameter of the pipette tips; the holes do not contract after the tips are removed since they are formed during PDMS thermal curing. As shown in inset of Figure 1. (d), the PDMS plug in the micropipette tip is tapered, which helps to produce clean holes when the micropipette tip is pulled out of PDMS.