Tamal Das
Department of Biotechnology, IIT Kharagpur-721302, India
Debapriya Chakraborty, Suman Chakraborty*
Department of Mechanical Engineering, IIT Kharagpur-721302, India
* e-mail: suman[at]mech.iitkgp.ernet.in
Why is this useful?
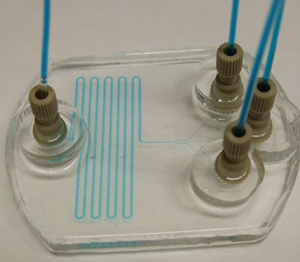
The integration of PDMS chips, with pumps or connections to reservoirs, has been found to pose serious difficulties because of its associated problems in leaking, interfacing with tubing, etc. Here, we present a simple approach for making reservoirs and also connecting devices with tubing for interfacing with pumps, without the aid of commercially available connectors and using commonly available consumables in the laboratory.
The PDMS mould is bonded with glass in two ways – using hydrophilic and hydrophobic means. Hydrophilic bonding involves using a plasma cleaner for oxidation. Hydrophobic bonding is done using a PDMS mixture. Here, we report easy and cost effective hydrophilic and hydrophobic bonding protocols. For example, the hydrophilic bonding can also be done without the use of conventional plasma oxidation.
What do I need?
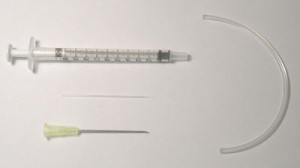
- PDMS base and curing agent (SYLGARD 184 silicone elastomer kit) in 10:1 wt/wt ratio
- micropipette tip (2-200 µl pipette tips)
- 20 gauge (outer diameter) needles
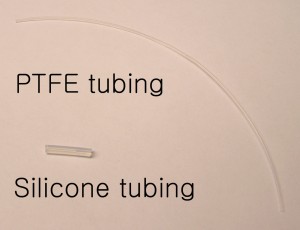
- tubing (20 gauge inner diameter)
- Piranha solution (H2O2 and H2SO4 solution in 1:1 vol/vol ratio)
What do I do?
1. Cut the syringe needles to the desired length (approximately 1.5 cm), preferably using wire electrical discharge machining. Any other needle cutter can be used if it can cut sharply the ends of the needles.
2. Punch holes in the PDMS mould using these needles at the inlet/outlet.
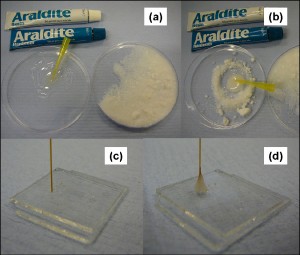
3. Bonding the PDMS mould to the substrate (Figure 2):
- Hydrophilic bond:
- Oxidize the glass slide and the PDMS mould in freshly prepared Piranha solution.
- Dip the glass slide in the solution for 5 mins, and the PDMS mould for 10 secs. Dipping the PDMS mould for larger times may lead to damage to the mould because of strong oxidation
- Rinse them in deionised water at least three times. This step is required to remove any residual acid as well as to prevent hydrophobic recovery.
- Dry both the glass slide and the mould using a compressed stream of dry nitrogen gas.
- Place the mould over the glass slide and heat it at 70°C for 30 minutes.
- Hydrophobic bond:
- Coat the glass slide with a thin layer (~ 10 µm thickness) of PDMS mixture (preferably by spin coater).
- Heat it at 70°C for 7 mins (this makes the PDMS hardened but still sticky)
- Place the PDMS mould on the sticky PDMS surface. In order to avoid wrinkles and air gaps, lay the mould down from one end to the other.
- Heat it for at least 30 mins at 70°C.
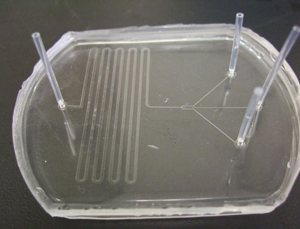
4. After bonding the PDMS mould to the glass, place the needles in the holes. Apply a small amount of PDMS mixture to the cylindrical surface of the needles. The PDMS mixture slowly settles down near the junction. Applying larger quantities of PDMS mixture may lead to seepage into the channel through the junction. Heat it immediately for 10-15 mins at 70°C. (Figure 3)
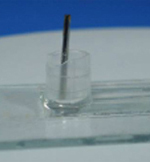
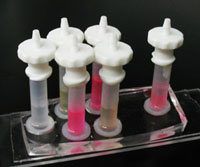
5. Cut a small piece of micropipette tip with at least one planar surface. Put a bit of PDMS mixture around the periphery of the planar surface. Place it centering the needles and heat it for 20 mins at 70°C. This makes the reservoirs. (Figure 4)
6. Pour PDMS mixture into the reservoirs around the syringe needle tips and heat it at 70°C for 25-30 mins until the supports become rigid. This provides the support for the needles. (Figure 5)
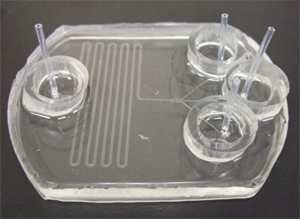
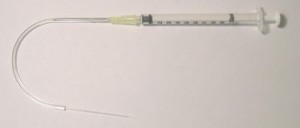
The tubing can be connected to these cut out needles, and the other ends connected to a syringe or peristaltic pump (Figure 6). If the connection to the pump is not desired and only the reservoirs are required then steps (1, 4, 6) are not required.