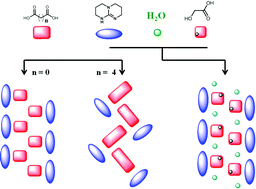
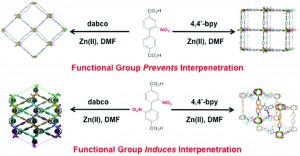
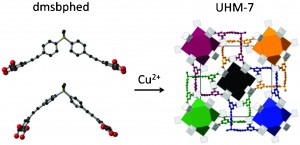
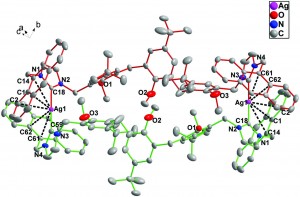
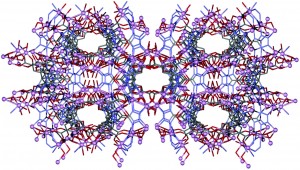
Supramolecular 1D ribbons in complexes between a bicyclic-guanidine derivative and di- or monocarboxylic acids
Vitthal N. Yadava and Carl Henrik Görbitz
CrystEngComm, 2013,15, 7321-7326
DOI: 10.1039/C3CE40960K
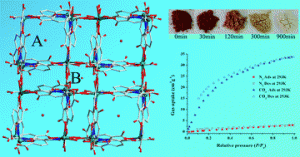
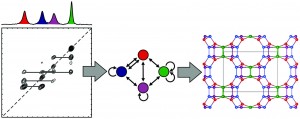
How to monitor guest exchange in host–guest systems
Luigi R. Nassimbeni and Hong Su
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40675J
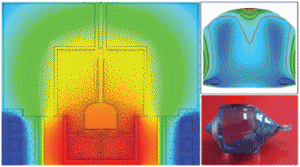
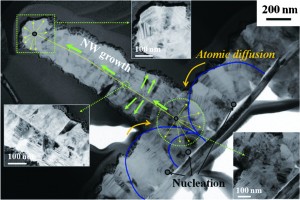
Nd:MgO:LiTaO3 crystal for self-doubling laser applications: growth, structure, thermal and laser properties
Dehui Sun, Yanhua Leng, Yuanhua Sang, Xueliang Kang, Shande Liu, Xiaoyong Qin, Kun Cui, Bin Kamaruddin Wan Hairul Anuar, Hong Liu and Yong Bi
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40966J
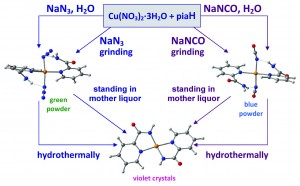
Iodine-templated assembly of an In(III) complex with a single-crystal-to-single-crystal transition
Yuan-Chun He, Wei-Qiu Kan, Jiao Guo, Yan Yang, Peng Du, Ying-Ying Liu and Jian-Fang Ma
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41337C
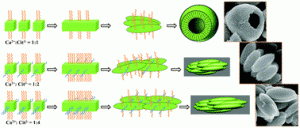
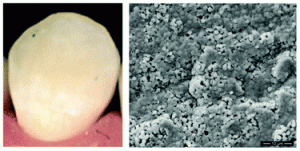
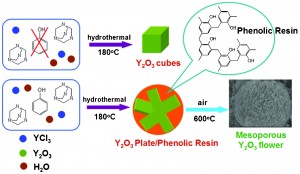
Effect of sodium citrate on the shape and photoluminescence properties of CaWO4:Eu3+ superstructures synthesized by the hydrothermal method
Yeqing Chen, Sung Wook Park, Byung Kee Moon, Byung Chun Choi, Jung Hyun Jeong and Chongfeng Guo
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40872H