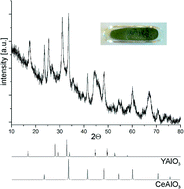
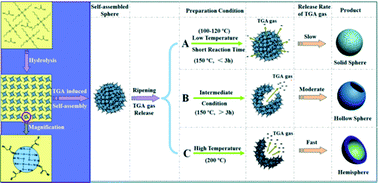
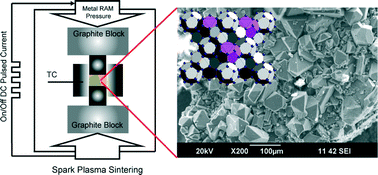
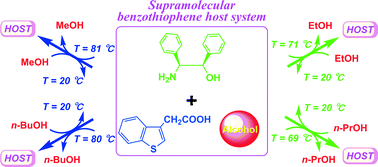
A follow up to the IUPAC project on the somewhat controversial issue on terminology of metal-organic frameworks and coordination polymers deals with the question of how we describe and communicate the structures of MOFs, PCPs and related network forming compounds.
The use of topology is very efficient in describing, disseminating and even designing such new materials, and the IUPAC task group deals with the project: Terminology guidelines and database issues for topology representations in coordination networks, metal-organic frameworks and other crystalline materials. As it turns out, network topology is also important, albeit sadly neglected, for the group 14 allotropes and even polymorphs of water.
The task group invite all who are interested to a workshop in Samara, Russia 21-23 May 2015 under the devise ”Describe, Disseminate and Design”. The workshop will feature talks by prominent scientist highlighting the network topology approach from many different angles, from mathematics to chemical synthesis and will also help the task group to gather viewpoints from the community. There will also be a poster session.
You can register and submit an abstract until 30th April 2015.
If you are interested but can’t make it, why not send thoughts directly to the project chair, Lars Öhrström or on twitter @larsohrstrom #IUPACmof2