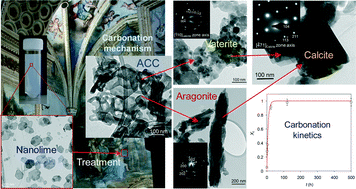
Nanolimes, alcoholic dispersions of colloidal Ca(OH)2 nanoparticles, are commonly used for the conservation of porous materials such as stone and marble. However, only the basics of the process they undergo – carbonation to produce CaCO3 – are understood and this limits their potential use.
A new paper by Rodriguez-Navarro looks at the carbonation process in detail, with the aims of increasing the effectiveness of and understanding any limitations in the use of nanolimes in conservation.
Conservation of materials using nanolimes is typically carried out in humid air at room temperature. Under these conditions, amorphous calcium carbonate (ACC) initially forms. This can then transform into the metastable vaterite (up to 35 wt%) and a small amount of aragonite (up to 5%), but only in the presence of alcohol. These polymorphs partially dissolve and the stable polymorph, calcite, precipitates. Alternatively, calcite can form directly after dissolution of ACC.
Results of the kinetic studies show that the rate-limiting step in the production of calcite is the amount of unreacted Ca(OH)2. Although the formation of metastable states might be considered a limitation to the use of nanolimes in conservation, the fast kinetics of the vaterite to calcite conversion (72 % in 10 days) means that almost the full consolidation potential can be reached within weeks of application and it is only over very short time-scales that the performance might be sub-optimal.
These results may also have implications for the design of new CaCO3 materials for other applications, using syntheses analogous to the multi-step crystallisation shown in the carbonation of nanolimes in the presence of alcohol.
For more information, read the full paper at:
Carlos Rodriguez-Navarro, Kerstin Elert and Radek Ševčík
CrystEngComm, 2016, Advance Article
DOI: 10.1039/C6CE01202G, Paper
__________________________________________________________________________________________________

Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She published a book, ‘Molecules, Medicines and Mischief’, in 2014, on some of the chemicals found in plants and is currently working on a follow-up.