The growth and design of organic crystals is often focused on linear regularity and the optical or electronic properties of the resulting crystals. Crystallisation is influenced by many factors including the presence of interactions between atoms or groups of atoms. However, when these are absent or insignificant, another kind of morphology can develop – the curved fractal. A new paper by Zhang et al. looks at the formation of a curved fractal structure and shows an application of these beyond the scientific realm.
Fractals are defined by the existence of the similar patterns occurring independent of the level of detail observed e.g. as seen in Koch snowflakes. A series of pyridyl diketones, with varying position of ring nitrogen and substitution, and a β-diketone were prepared and their properties compared.
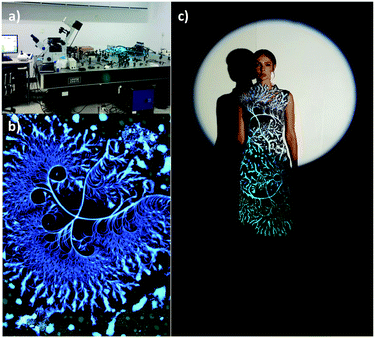
The luminescent 1-(4-methoxyphenyl)-3-(pyridin-2-yl)propane-1,3-dione (PBDK1) showed, uniquely among the structures studied, a curved fractal structure visible using an electron microscope (seen below in fluorescence mode). The highest curvature was observed for crystals obtained by evaporation from a solution in acetone at 5.0 mg mL−1 or above, on glass. Under these conditions, the relatively weak (compared with the 3- and 4-pyridyl analogues) potential intermolecular interactions in PBDK1 can be disrupted by surface tension effects. This allows molecular slippage and dislocation to occur during crystal growth. The observed curved fractal pattern was transferred onto fabric and the design incorporated in a dress which won first place at the 2016 National Women’s Fashion Design Competition of China.
 This work suggests some of the factors involved in the formation of curved fractal structures and also shows that interest in these extends beyond the scientific and into the realm of fashion design.
This work suggests some of the factors involved in the formation of curved fractal structures and also shows that interest in these extends beyond the scientific and into the realm of fashion design.
For more information, read the paper at:
Curved fractal structures of pyridine-substituted β-diketone crystals
Zongzheng Qian, Dongxue Li, Tongqing Xie, Xuepeng Zhang, Yang He, Yuejie Ai and Guoqing Zhang
DOI: 10.1039/C7CE00462A
________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She published a book, ‘Molecules, Medicines and Mischief’, in 2014, on some of the chemicals found in plants and is currently working on a follow-up.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She published a book, ‘Molecules, Medicines and Mischief’, in 2014, on some of the chemicals found in plants and is currently working on a follow-up.