Posted on behalf of Josh Campbell, web writer for CrystEngComm
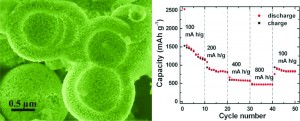
Lithium ion batteries (LIB) are ubiquitous in modern life. Consumer electronics generally use LIBs based on LiCoO2 but these have safety drawbacks and environmental concerns. NiO has been proposed as a replacement material for the anode due to its safety, low cost and theoretical capacity. However bulk NiO has poor electrochemical performance and much research has been focused on developing nanostructures that would allow NiO to reach its full potential. Porous hollow materials can offer many improvements compared to the bulk such as improved capacity and cycling performance.
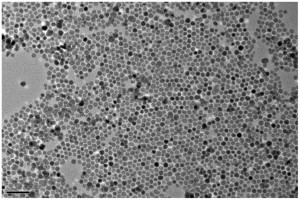
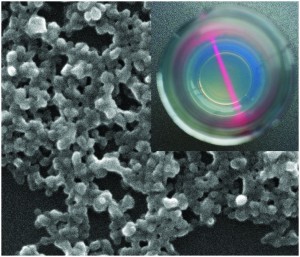
A new paper presents the synthesis of NiO porous hollow microspheres using L-cysteine as a directing agent. The directing agent causes the spheres to form, as without it the precursors to NiO grow into nanoplate-assembled flowers. The authors propose a mechanism for the sphere formation: L-cysteine complexes with Ni(OH)2, which during hydrothermal treatment aggregate due to the hydrophobic interactions of the L-cysteine. These continue to aggregate throughout treatment and assemble into spheres. The NiO microspheres showed improved reversibility and good capacity retention. The authors attribute the improved performance to the hollow architecture which allows for fast ion/electron transfer.
Find out more from the paper:
L-cysteine-assisted preparation of porous NiO hollow microspheres with enhanced performance for lithium storage
Dong Xie, Qingmei Su, Zimin Dong, Jun Zhang and Gaohui Du
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41161C, Paper
 Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.
Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.