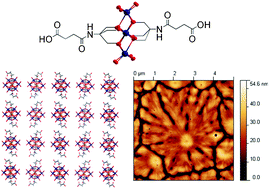
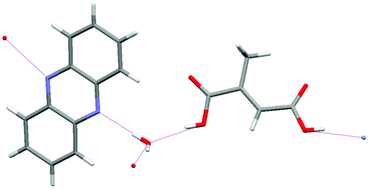
The Gamez group have analyzed halogen–π interactions by a statistical study of the occurrence and directionality of the interactions in the solid-state. They used structures from the Cambridge Structural Database (CSD) in combination with an analytical procedure developed and used previously by the authors for other important interactions like lone pair–π and anion–π interactions. The results clearly demonstrate that halogen bonding to a phenyl ring are directional, and could compete with hydrogen bonding. Read more about competition and directionality in the solid state for FREE at:
Halogen-phenyl supramolecular interactions in the solid state: hydrogen versus halogen bonding and directionality
Tiddo J. Mooibroek and Patrick Gamez
CrystEngComm, 2013, 15, 1802-1805
DOI: 10.1039/C2CE26853A
Other related reading from the same group includes:
Anion–arene and lone pair–arene interactions are directional
Tiddo J. Mooibroek and Patrick Gamez
CrystEngComm, 2012, 14, 1027-1030
DOI: 10.1039/C1CE05946G
How directional are D–H phenyl interactions in the solid state (D = C, N, O)?
Tiddo J. Mooibroek and Patrick Gamez
CrystEngComm, 2012, 14, 8462-8467
DOI: 10.1039/C2CE26205C
You may also be interested in:
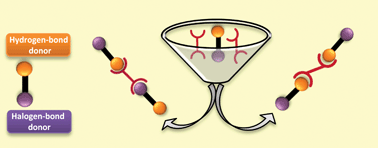
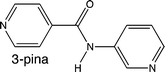
Competing hydrogen-bond and halogen-bond donors in crystal engineering
Christer B. Aakeröy, Sheelu Panikkattu, Prashant D. Chopade and John Desper
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C2CE26747K
or also on our blog
Follow us on Twitter: @crystengcomm