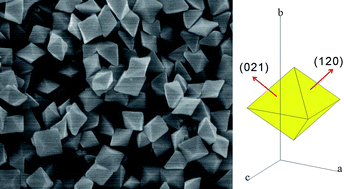
Mandi Han and colleagues at the Nanyang Technological University in Singapore have synthesised octahedral single crystals of monoclinic bismuth vanadate (m-BiVO4) by hydrothermal methods. Reaction times, acid concentration and the addition of the surfactant sodium dodecyl benzene sulfonate all influence the growth of good crystals. Electron microscope measurements reveal the uniform size of these octahedral single crystals and preferred {120} and {021} crystalline facets. The m-BiVO4 crystals exhibit excellent photocatalytic performance determined by the degradation of rhodamine B under visible light irradiation. Future research will investigate the effect of different facets on photocatalysis performance.
Read the full paper to find out more…
Synthesis of mono-dispersed m-BiVO4 octahedral nano-crystals with enhanced visible light photocatalytic properties
Mandi Han, Xiaofeng Chen, Ting Sun, Ooi Kiang Tan and Man Siu Tse
CrystEngComm, 2011, Advance Article
DOI: 10.1039/C1CE05539A











