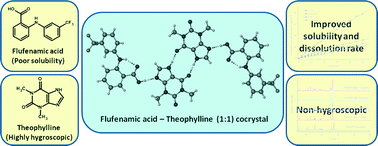
Flufenamic acid (FFA) is a potent non-steroidal anti-inflammatory drug used to treat lower back pain, either orally or topically. However, it has low solubility and a slow dissolution rate which are problematic. One way these properties can be improved is by forming a solid solution with polyvinylpyrrolidine or a co-crystal with nicotinamide (NA). Co-crystals form between the active molecule and a co-former and possess different physicochemical properties from either of the components.
A recent CrystEngComm article reports the formation and X-ray structures of three new co-crystals of FFA, with the co-formers theophylline (TP), 4-pyridone and 4,4’-bipyridine. Each of the three structures shows the formation of heterosynthons (i.e. interactions between complementary functional groups) e.g. for FFA-TP, interactions between FFA O-H and TP N atoms. TP is a central nervous system stimulant, found in cocoa beans, and used to treat respiratory diseases such as asthma. The FFA-TP system is an example of a drug-drug co-crystal, where both components have biological activity and therefore is of particular interest.
The authors found improved solubility and dissolution rate for this system, compared to either pure FFA or the FFA-NA co-crystal. They also compared the properties to a TP co-crystal with oxalic acid, with the FFA-TP co-crystal proving less hygroscopic.
These results suggest that formation of this co-crystal offers improved properties for both FFA and TP and in addition, offers an opportunity to explore the development of combination drugs by forming drug-drug co-crystals.
For more information see the paper:
DOI: 10.1039/C3CE42182A, Paper
____________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants.