Iron vanadate (FeVxOy) nanostructures have shown very good performance in sensors, lithium batteries and as catalysts. Their properties are strongly related to the shape and surface area of the particles and this makes the controllable preparation of one dimensional (1D) nanostructures (i.e. nanowires or nanorods), with large surfaces areas, a target for scientists.
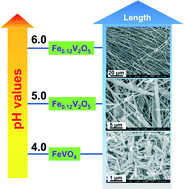
A new paper reports a simple hydrothermal technique which achieves this. The length of the particles can be adjusted simply by varying the pH of the reaction mixture between pH 4 and pH 6, with longer wires favoured at higher pH, as shown in the diagram below. Using this methodology, lengths from several micrometers to several millimetres can be obtained and the ratios of diameters to lengths can also be varied from 10 to over 1000. In addition, the pore sizes in the nanostructures can also be controlled using the same method of pH variation.
There are four steps in the formation of the nanostructures – dissolution, anisotropic growth (i.e. growth in one direction), Ostwald ripening (a process where smaller particles dissolve and deposit on larger particles to achieve more thermodynamically stable particles) and, finally, pore formation by loss of water molecules.
A sample of one of the prepared nanostructures (FeVO4 nanorods) was tested for use in selective catalytic reduction (SCR) of NO with NH3 as the reduction of NOx emissions from diesel engines is important to reduce air pollution. The nanorods proved stable and selective under typical reaction conditions and, in addition, were resistant against two major catalyst poisons present in exhaust fumes, H2O and SO2.
For more information, read the full paper using the link below:
Hydrothermal growth and characterization of length tunable porous iron vanadate one-dimensional nanostructures
Lei Huang, Liyi Shi, Xin Zhao, Jing Xu, Hongrui Li, Jianping Zhang and Dengsong Zhang
CrystEngComm, 2014, DOI: 10.1039/C3CE42608D
_______________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently, she is writing a book on chemicals from plants