 Fluorine is special. It is uniquely characterized by its high electronegativity, relatively small size, very low
Fluorine is special. It is uniquely characterized by its high electronegativity, relatively small size, very low
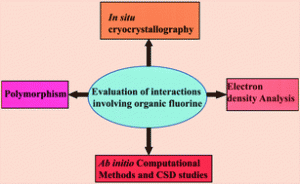
polarizability of the bound three nonbonding electron pairs and excellent overlap between fluorine 2s and 2p orbitals with corresponding orbitals of second row elements. In this CrystEngComm Highlight Deepak Chopra and Tayur Guru Row look at how the special attibutes of Fluorine affect the crystal lattice building of organic fluorine compounds.
Role of organic fluorine in crystal engineering
Deepak Chopra and Tayur N. Guru Row
CrystEngComm, 2011, DOI: 10.1039/C0CE00538J, Highlight










