A recent paper in CrystEngComm details a new synthetic method to create magnenite (iron oxide) nanoparticles (NPs), based on the orientated aggregation of particles using a calixarene macrocycle. The new method allows for control of the type of NPs produced and could potentially be applied to the growth of other nanomaterials.
Magnetite has promising biomedical applications due to its interesting magnetic and electrochemical properties and has been studied for drug delivery, biosensing and cancer treatment. The NPs can reduce oxidative stress from electromagnetic radiation and raise temperature around the tumour cells in the presence of an applied magnetic field, killing cancer cells through localised heating. In contrast to traditional strategies of crystal growth, orientated aggregation is a mediated approach in which primary crystallites assemble into secondary crystals. The mediator can be other particles, specific molecules or an applied field. NPs grown this way often exhibit different morphologies and properties to the starting material.
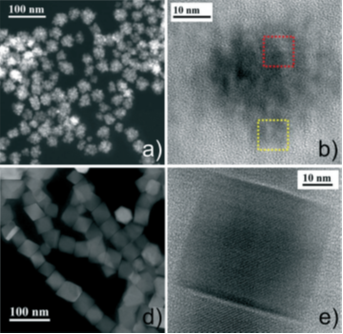
The authors postulated that the use of an organic molecule that complexes with iron could be used to induce aggregation and chose p-tert-butylcalix[8]arene, calixarene molecules are used in host-guest chemistry to induce efficient and selective hosts. A reaction mixture of the calixarene, iron(III) acetylacetonate and oleic acid was heated at 200°C for 2 hours, before being refluxed for one hour to promote crystal growth. The initial ratio of the reactants (0.5:1:2) produced multicore NPs with an average size of 40nm and primary crystallites of 7nm. Raising the iron:calixarene ratio to 1:1 resulted in a new morphology being produced, octahedral NPs were now produced exclusively with an average size of 50nm. An experiment without the calixarene produced different NPs again, this time much smaller (7nm) single core NPs. Further experiments varying the heating time and ratios resulted in defect octahedral NPs.
The colloidal and magnetic properties of the NPs were then measured with the multicore structures exhibiting excellent properties. The authors conclude that their synthesis method is easy to perform, reproducible and controllable.
For more information, read the full paper at:
Francesco Vita, Helena Gavilán, Francesca Rossi, César de Julián Fernández, Andrea Secchi, Arturo Arduini, Franca Albertini and M. Puerto Morales
DOI: 10.1039/C6CE01252C