Nitrobenzene is an important industrial material used in the production of aniline, dyes, pharmaceuticals and explosives. However, it is highly toxic, considered likely to be a human carcinogen and does not readily degrade so accurate sensing of nitrobenzene in the environment, even at very low concentrations, is required.
A new paper presents a Cd-based metal-organic framework (MOF) as a highly selective and efficient sensor of nitrobenzene. The MOF is based on a new flexible ligand with aromatic character, chosen to complement nitrobenzene’s electron withdrawing character. The ligand, 1,1-(1,4-phenylenebis(methylene))bis(1H-pyrazole-3,5-dicarboxylicacid) and a source of Cd react under hydrothermal conditions to form the new Cd-MOF.
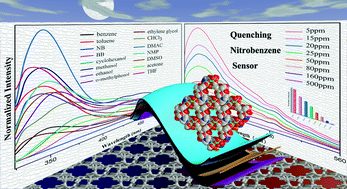
This shows a fluorescence emission when excited at 297 nm which is quenched by the presence of nitrobenzene (see diagram below).
The suggested mechanism of quenching involves photoinduced electron transfer. Quenching is inversely related to the concentration of nitrobenzene and significant quenching occurs at concentrations as low as 50 ppm nitrobenzene. Thus, Cd-MOF is suggested as a sensitive, rapid probe for low concentrations of nitrobenzene.
For the full paper see:
A new Cd(II)-based metal–organic framework for highly sensitive fluorescence sensing of nitrobenzene
Yu-Pei Xia, Yun-Wu Li, Da-Cheng Li, Qing-Xia Yao, Yu-Chang Du and Jian-Min Dou
CrystEngComm, 2015, Advance Article
DOI: 10.1039/C5CE00162E
__________________________________________________________________________________________________

Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.