Control of the crystal form of pharmaceutically important molecules such as paracetamol is crucial to the successful development of drug molecules. Conventional crystallisation from organic solvents can lead to unwanted forms with poor physicochemical properties. Crystallisation from ionic liquids (ILs) offers a potential alternative. ILs are composed entirely of ions and have low melting points as their cationic components are large and unsymmetrical, resulting in low lattice energies.
A new paper shows how the crystallisation of paracetamol, commonly used to reduce pain and fever, from ILs can be controlled. Use of two ILs, 1-hexyl-3-methylimidazolium hexafluorophosphate ([hmim][PF6]) and 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim][PF6]) was studied, under cooling crystallisation conditions.
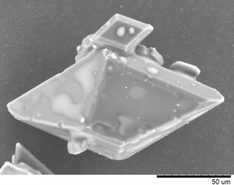
The thermodynamically stable monoclinic I form of paracetamol was obtained from both ILs but the crystal size and shape varied with the IL used, the solution concentration and the mechanism of crystal growth. One of the samples produced is shown below.
Crystal habits not commonly produced by conventional crystallisation could be produced – elongated prisms from [bmim][PF6] and trigonal bipyramids from [hmim][PF6]. These results suggest that ILs have potential value for the crystal engineering of pharmaceutically important molecules.
For full details, see the paper at:
Crystallisation control of paracetamol from ionic liquids
K. B. Smith, R. H. Bridson and G. A. Leeke
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C4CE01796J
___________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.