We have a new crop of HOT articles which are free to access for 4 weeks. These have also been compiled into a collection and are available for viewing on our website.
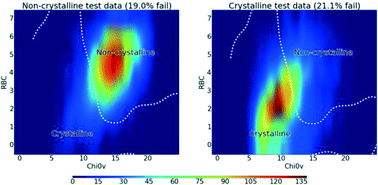
Will it crystallise? Predicting crystallinity of molecular materials
Jerome G. P. Wicker and Richard I. Cooper
CrystEngComm, 2015, Advance Article
DOI: 10.1039/C4CE01912A
Free to access until 28th December 2014
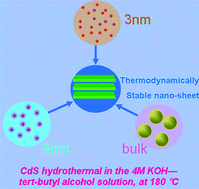
New evidence of a thermodynamically stable nanophase: CdS in 4 M KOH–tert-butanol solution
Jinsheng Zheng, Xiaogang Xue, Dongsong Li and Yibing Zhao
CrystEngComm, 2015, Advance Article
DOI: 10.1039/C4CE01901F
Free to access until 28th December 2014
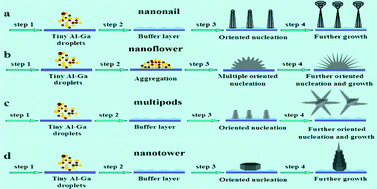
Morphology-controlled synthesis and structural characterization of ternary AlxGa1−xN nanostructures by chemical vapor deposition
Fei Chen, Xiaohong Ji and Qinyuan Zhang
CrystEngComm, 2015, Advance Article
DOI: 10.1039/C4CE01886A
Free to access until 28th December 2014
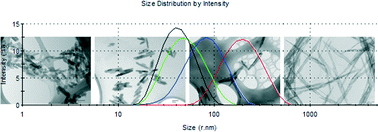
Tuning the size and shape of nano-boehmites by a free-additive hydrothermal method
Pablo Pardo, Noemí Montoya and Javier Alarcón
CrystEngComm, 2015, Advance Article
DOI: 10.1039/C4CE02094D
Free to access until 28th December 2014












 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.