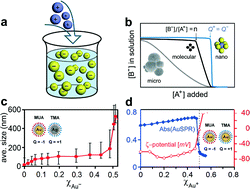
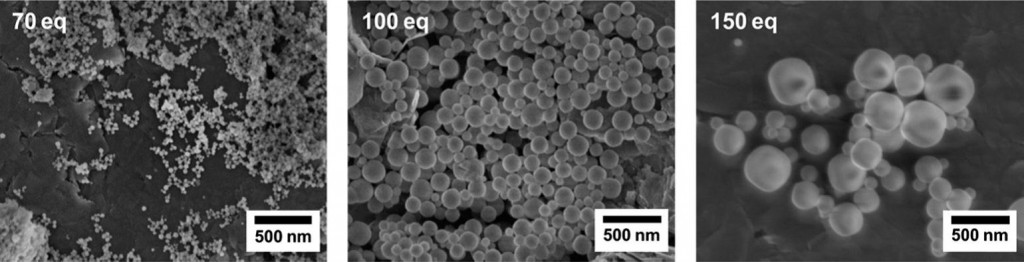
The Royal Society of Chemistry is proud to jointly host the International Conference on Structural Chemistry of Molecules and Materials (SCOMM14) with the University of Calcutta, Jadavpur University and IISER-Kolkata.
It will take place at the Center for Research in Nanoscience and Nanotechnology (CRNN), University of Calcutta from the 30th November to 2nd December 2014.

The conference is being organized to celebrate the International Year of Crystallography (IYCr) and covers contemporary problems of crystal engineering, materials synthesis, chemical structure and dynamics.
Confirmed Speakers
Professor Gautam Desiraju (IIS Bangalore)
Professor Kumar Biradha (IIT Kharagpur)
Professor Neil Champness (University of Nottingham)
Professor Susan Bourne (University of Cape Town)
Professor Len MacGillivray (University of Iowa)
Professor Russell Morris (University of St Andrew’s)
Professor Chilla Malla Reddy (IISER Kolkata)
Professor George Shimizu (University of Calgary)
Professor Michael Ward (University of Sheffield)
It will focus on all aspects of structural chemistry, including multidisciplinary areas, and will offer the opportunity for scientists from many different countries to exchange their scientific experience as well as to intensify their cooperation to partners.
For full details of confirmed speakers, venue information, and registration. See the website.











 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.