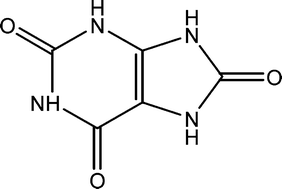
Human kidney stones contain over 200 components, most significantly, anhydrous uric acid (UA, below) and its dihydrate (UAD). When UAD is present, UA is also, however the reverse isn’t always true. This suggests the conversion of UAD to UA may be a significant step in the formation of kidney stones. A deeper understanding of how the stones form could inform strategies to prevent their formation and to disperse them once formed.
A new paper in CrystEngComm looks at the relationship between UAD and UA under physiologically relevant conditions. The authors studied the behaviour of UAD in aqueous solution at body temperature (37oC), both at various pHs in the presence of a buffer and in an artificial urine solution. In aqueous solution at acidic pH values, the conversion of UAD occured via a slow dissolution, followed by recrystallization, to form UA over 42 hours. At neutral pH, the final product formed was uric acid monohydrate (UAM), which was obtained either directly or via a UA intermediate. In urine solution, UA formation was much faster (complete in 30 hours) and crystals were much smaller.
The rate limiting step is believed to be the dissolution of UAD, with the timescale of the UA formation explaining why UAD is rarely found in the absence of UA. Future studies will look at how other urinary components and/or additives can affect UA formation.
For more information, see the full paper:
Solution-mediated phase transformation of uric acid dihydrate
Janeth B. Presores and Jennifer A. Swift
CrystEngComm, 2014, DOI: 10.1039/C4CE00574K
_______________________________________________________________________________________________________
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. She has recently published a book on chemicals from plants.