In this Editor’s choice post, Professor Nicola Pinna, a member of the CrystEngComm Editorial Board, talks about his favourite articles published in the journal in recent months. Nicola has chosen the most interesting articles in the area of controlled growth of nanostructured materials.
Mechanistic analyses of nanoscale transformations are not easily accessible, however this case reported by Hoshyargar and co-workers is an exception as the authors managed to follow the transformation of tungsten oxide nanostructures to WS2 hollow particles. The starting material was synthesized by my favourite approach: a simple, solvothermal treatment of a tungsten alkoxide in benzyl alcohol, with the well-defined tungsten oxide nanoplatelets being subsequently converted to WS2 by sulfidization at higher temperature. The growth mechanism was investigated by applying advanced electron microscopy techniques at different stages of the solid state reaction. The final product, the “hollow rectangular WS2 nanoboxes”, was shown to be formed by “a cascade” of topotactic and epitactic processes. What I found the most astonishing was the last step of the mechanism, involving the fusion of the layered WS2 sheets forming almost perfect rectangular boxes with 90° kinks (see figure below).
Unconventional upright layer orientation and considerable enhancement of proton–electron conductivity in Dion–Jacobson perovskite thin films
Tomohiko Nakajima, Kiyoshi Kobayashi, Kentaro Shinodaa and Tetsuo Tsuchiyaa
CrystEngComm, 2014, 16, 4113-4119
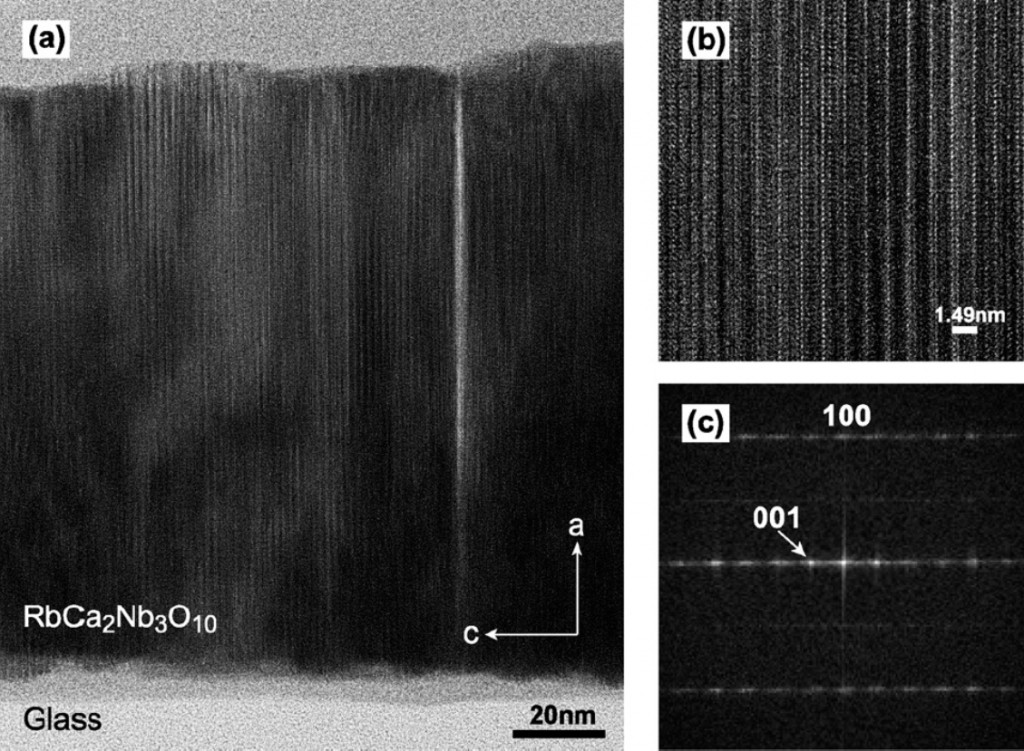
Single crystal substrates are generally needed for growth of single-crystalline and highly-oriented oxide thin films. However, such an approach cannot be used in industrial applications for which the growth of highly-oriented oxide thin films should take place on common substrates. In this article, Nakajima et al. show that by pulsed laser-assisted annealing of an oxide precursor thin film that is spin coated from a molecular precursor solution, single crystalline RbCa2Nb3O10 layered perovskite thin films can be obtained. The peculiarity of these films is that they show upright layer orientations (see figure below) which may allow for them to be used as membranes for fuel cells due to the potential for high proton conductivity in the interlayer sites. The unusual orientation of the films is possibly connected to oxygen deficiencies near the surface which as caused by the high laser power needed to obtain the upright layer orientation.
Layered titanosilicates for size- and pattern-controlled overgrowth of MFI zeolite
Stanislav Ferdov
CrystEngComm, 2014, DOI: 10.1039/C3CE42644K
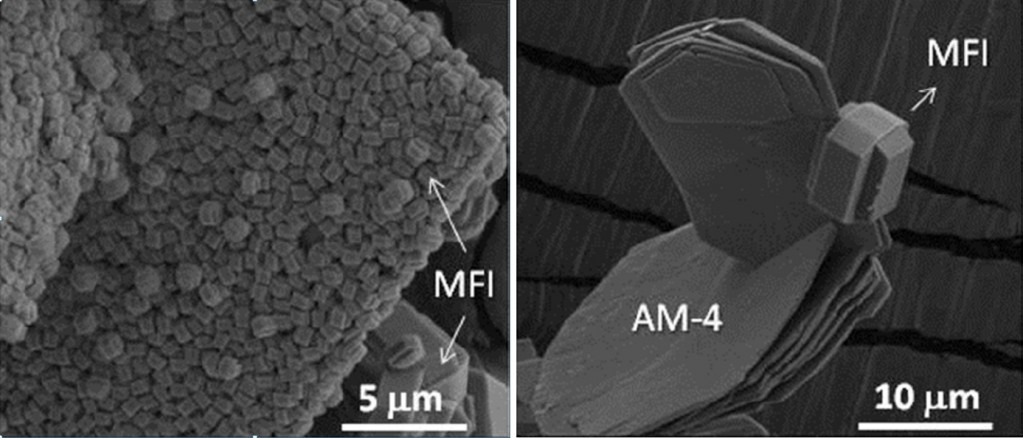
There is a need for controlling the growth of zeolites on different substrates which can only be achieved by understanding of the heterostructural interconnections between the support and the zeolite material. This is particularly relevant for industrial applications such as catalysis. In this work, Stanislav Ferdov reports that plate-like crystals of two titanium silicates can act as interesting substrates for the controlled growth of an MFI-type zeolite. The different arrangement of the SiO4 tetrahedra and TiO6 octahedra in the two chosen titanosilicates govern the overgrowth behaviour. In one case, the plate-like crystals of the titanosilicate (20–40 μm) are covered with a monolayer of regularly packed-zeolite crystals (see figure below left). In the second case, the support acts as a single-nucleation site and only isolated and larger crystals of MFI zeolite are observed (see figure below right). Although the possibility for controlling the overgrowth of zeolites has clearly been demonstrated, the opportunity for improvement is huge. It can only be successful, however, if combined with a molecular-scale understanding of the heterostructural interconnections between substrate and zeolite.
Ligand dynamic effect on phase and morphology control of hexagonal NaYF4
Suli Wu, Ye Liu, Jie Changa and Shufen Zhang
CrystEngComm, 2014, DOI: 10.1039/C4CE00109E
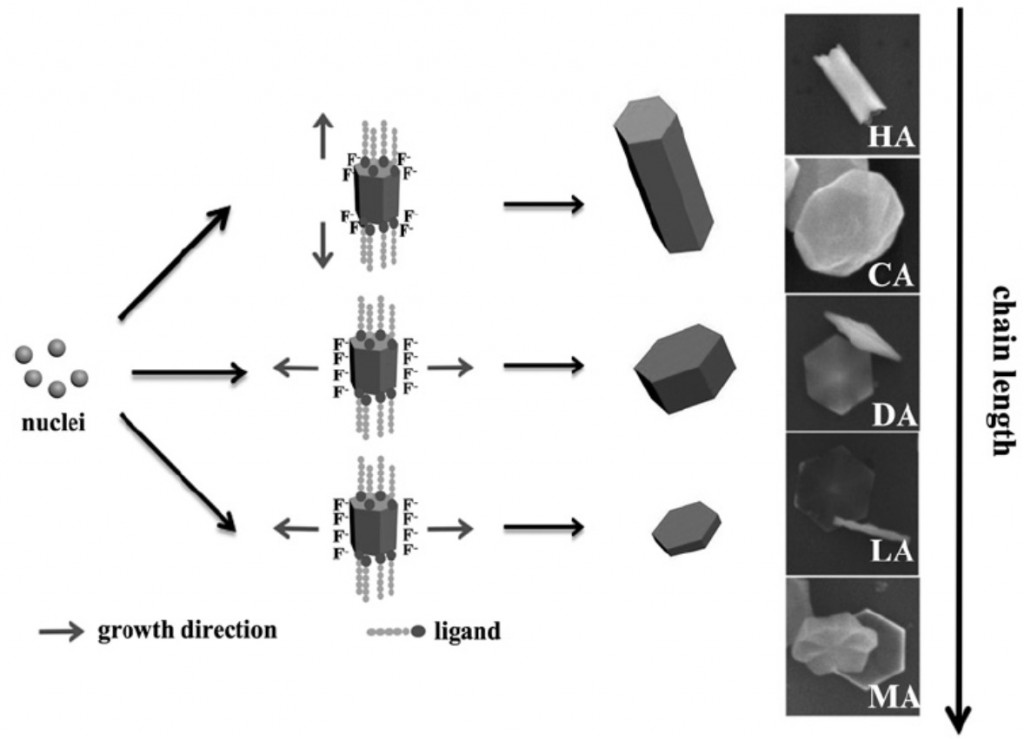
It is well accepted that the adsorption of stabilizing ligands at the surface of nuclei dramatically influences the growth habit of inorganic nanocrystals. The organic–inorganic interface present in these systems is the key to the synthesis of morphology controlled nanocrystals. Ligands can selectively adsorb onto particular facets hindering the growth in the direction perpendicular to that facet. However, the adhesion energy of the ligands should be adjusted to allow dynamic adsorption-desorption during nanoparticle growth, as demonstrated in the past for the synthesis of quantum dots. More importantly, it has also been demonstrated that, for the same functional group, the dynamic effect on the surface of quantum dot nanocrystals is significantly influenced by the ligand chain length. In this article, the authors investigate the effect of the alkyl chain length of carboxylic acid ligands on the growth of NaYF4 nanocrystals. The much more mobile hexanoic acid (HA) does not effectively passivate the {001} facets leading to rod-shape nanoparticles. With longer alkyl chains ligands, the growth along the c-axis is decreased (see figure below) and the morphology can be almost continuously tuned from rods to very thin disk-shaped crystals. The authors attribute such precise morphology control to the different ligand dynamic as, for longer alkyl chain ligands, the tendency of leaving the (001) crystal surfaces decreases leading to a lower growth on the [001] direction.
 Nicola Pinna studied physical chemistry at the Université Pierre et Marie Curie (Paris). He received his Ph.D. in 2001 and in 2002 moved to the Fritz Haber Institute of the Max Planck Society (Berlin). In 2003, he joined the Max Planck Institute of Colloids and Interfaces (Potsdam) before moving to the Martin Luther University, Halle-Wittenberg, as an Assistant Professor of Inorganic Chemistry in 2005. From March 2006 to June 2012 he was researcher at the Department of Chemistry and CICECO of the University of Aveiro and from September 2009 to June 2012 he was also Assistant Professor at the school of chemical and biological engineering Seoul National University. In July 2012 he joined the Department of Chemistry of the Humboldt University in Berlin. His research activity is focused on the development of novel routes to nanostructured materials, their characterization, and the study of their physical properties.
Nicola Pinna studied physical chemistry at the Université Pierre et Marie Curie (Paris). He received his Ph.D. in 2001 and in 2002 moved to the Fritz Haber Institute of the Max Planck Society (Berlin). In 2003, he joined the Max Planck Institute of Colloids and Interfaces (Potsdam) before moving to the Martin Luther University, Halle-Wittenberg, as an Assistant Professor of Inorganic Chemistry in 2005. From March 2006 to June 2012 he was researcher at the Department of Chemistry and CICECO of the University of Aveiro and from September 2009 to June 2012 he was also Assistant Professor at the school of chemical and biological engineering Seoul National University. In July 2012 he joined the Department of Chemistry of the Humboldt University in Berlin. His research activity is focused on the development of novel routes to nanostructured materials, their characterization, and the study of their physical properties.