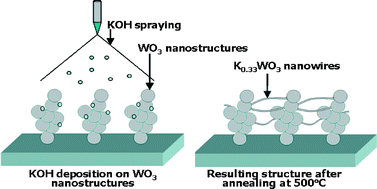
Connectivity enhancement of highly porous WO3 nanostructured thin films by in situ growth of K0.33WO3 nanowires
Julien Gaury, Ugo Lafont, Eugene Bychkov, Andreas Schmidt-Ott and George Biskos
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42078G
Free to access until 17th January 2014
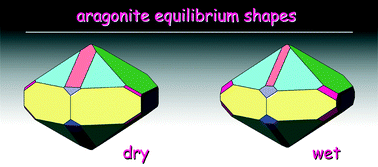
Surface structure, morphology and (110) twin of aragonite
Francesco Roberto Massaro, Marco Bruno and Marco Rubbo
CrystEngComm, 2014,16, 627-635
DOI: 10.1039/C3CE41654B
Free to access until 17th January 2014
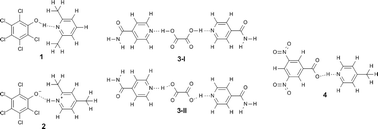
Determining hydrogen positions in crystal engineered organic molecular complexes by joint neutron powder and single crystal X-ray diffraction
Marc Schmidtmann, Paul Coster, Paul F. Henry, Valeska P. Ting, Mark T. Weller and Chick C. Wilson
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42070A
Free to access until 17th January 2014
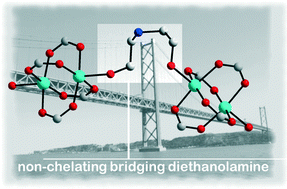
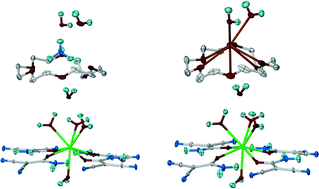
How to Force a Classical Chelating Ligand to a Metal Non-Chelating Bridge: the Observation of a Rare Coordination Mode of Diethanolamine in the 1D Complex {[Cu2(Piv)4(H3tBuDea)](Piv)}n
Oksana V. Nesterova, Marina V. Kirillova, M. Fátima C. Guedes da Silva, Roman Boca and Armando J. L. Pombeiro
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE41657G
Free to access until 3rd January 2014
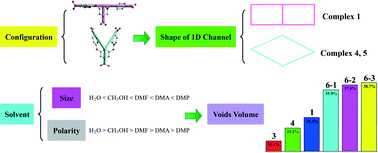
The influence of ligand configuration, solvent size and solvent polarity on the porous shape and void volume in a series of isomeric or isomorphic porous MOFs
Wen-Huan Huang, Xin-Jun Luan, Xiang Zhou, Jun Chen, Yao-Yu Wang and Qi-Zhen Shi
CrystEngComm, 2013,15, 10389-10398
DOI: 10.1039/C3CE41801D
Free to access until 3rd January 2014
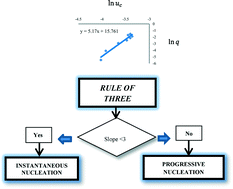
Determination of the nucleation mechanism and kinetics from the analysis of polythermal crystallisation data: methyl stearate from kerosene solutions
Diana M. Camacho Corzo, Antonia Borissova, Robert B. Hammond, Dimo Kashchiev, Kevin J. Roberts, Ken Lewtas and Iain More
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE41098F
Free to access until 3rd January 2014
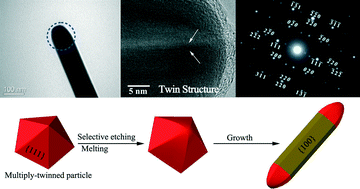
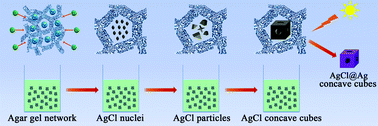
Silver nanowires with rounded ends: ammonium carbonate-mediated polyol synthesis, shape evolution and growth mechanism
Shaohong Liu, Boming Sun, Ji-guang Li and Jialin Chen
CrystEngComm, 2014,16, 244-251
DOI: 10.1039/C3CE41738G
Free to access until 3rd January 2014
Intermolecular contacts in bromomalonic aldehyde—intuition, experiment, and theory
Volker L. Deringer, Fangfang Pan, Janine George, Paul Müller, Richard Dronskowski and Ulli Englert
CrystEngComm, 2014,16, 135-138
DOI: 10.1039/C3CE41779D
Free to access until 3rd January 2014
A Neutron Diffraction Study of Hydrogen Bonding in Isostructural Potassium and Ammonium Lanthanoidates
Adrian J. Emerson, Alison J. Edwards, Stuart R. Batten and David R. Turner
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE42031K
Free to access until 3rd January 2014
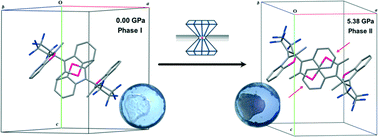
High-Pressure Crystallographic and Spectroscopic Studies on Two Molecular Dithienylethene Switches
Christopher H. Woodall, Simon K. Brayshaw, Stefanie Schiffers, David R. Allan, Simon Parsons, Rafael Valiente and Paul R. Raithby
CrystEngComm, 2014, Advance Article
DOI: 10.1039/C3CE41933A
Open access![]()













 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she is writing a book on chemicals from plants.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she is writing a book on chemicals from plants.