Posted on behalf of Gwenda Kyd, web writer for CrystEngComm
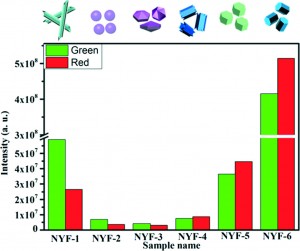
Controlling the size and shape of crystals is useful when these factors are related to properties of interest, such as luminescence. In the case of hexagonal sodium yttrium fluoride (β-NaYF4), the upconversion luminescence (i.e. conversion of longer wavelength infra-red radiation to shorter wavelength visible light) varies with the size and morphology of the microcrystals.
A new paper shows how the morphology can be conveniently and predictably varied by altering the ratio of NaF to RE 3+ (where RE3+ is the total amount of Y3+ and dopant Yb3+ and Er3+) in a simple hydrothermal synthesis. Changing this ratio leads to the formation of tubes, spheres, rods, bipyramids, plates and prisms of different sizes. In the paper, the authors also suggested a potential mechanism for formation of the different morphologies. As shown in the figure below, the upconversion emissions of green and red light vary for these morphologies.
For more details see the full paper:
Controllable synthesis, formation mechanism and upconversion luminescence of β-NaYF4 : Yb3+/Er3+ microcrystals by hydrothermal process
Mingye Ding, Chunhua Lu, Linhai Cao, Yaru Ni and Zhongzi Xu
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41427B, Paper
 Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she works as a scientific database editor.
Gwenda Kyd has a PhD in metallocarborane chemistry from the University of Edinburgh. Other research work includes the spectroscopic study of the structure of glasses and organometallic electron-transfer reactions and the preparation of new inorganic phosphors. Currently she works as a scientific database editor.