Posted on behalf of Josh Campbell, web writer for CrystEngComm
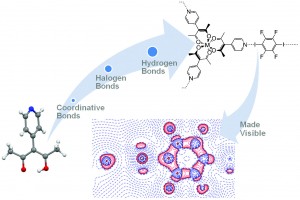
Ditopic ligands (ligands capable of coordination at two separate sites) allow the creation of well-ordered extended complexes containing different cations. They are usually N-, P-, O-, and S-containing (or in their N, O-, N, S,- and N, P-combinations) organic molecules, and have been used in various applications such as monitoring guest exchange and the creation of metal organic frameworks. The multi-centre nature of these ligands allows for other interactions outside of coordinative and hydrogen bonding such as halogen bonding. 3-(4-pyridyl)-2,4-pentanedione (HacacPy) is a well known ditopic ligand, and in this new work, has been used to create a crystal in which these three types of bonding are represented.
Three complexes containing HacacPy and tetrafluorodiiodobenzene (TFDIB) were prepared. Compound 1 showed the halogen bonding produced between the pyridine N and the iodine of TFDIB with this being the only coordination centre used. In compound 2 HacacPy is deprotonated and is involved in coordinative bonding using the acac part of the ligand and two pyridine N atoms form halogen bonds to TFDIB producing chains. Compound 3 introduces a third interaction, hydrogen bonding of a Py N to a solvent molecule which is in turn halogen bonded to a TDIB which is halogen bonded to another Py N. The authors analysed the charge density of 3 and provided the first tentative experimental results of the effect of metal coordination on halogen bonds.
Find out more from the paper:
3-(4-Pyridyl)-2,4-pentanedione – a bridge between coordinative, halogen, and hydrogen bonds
Carina Merkens, Fangfang Pan and Ulli Englert
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE41306C, Paper
 Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.
Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.