Posted on behalf of Josh Campbell, web writer for CrystEngComm
Metal nanoparticles (NPs) are structures within the µm-nm range that exhibit amazing properties and morphologies. Photochemical and superparamagnetic effects have been reported while everything from nanostars to nanoreefs have been created. NPs are often thought to be a product of modern science but their use dates back hundreds of years, used in pottery finishes and for staining glass. Michael Faraday first described the effect of gold colloids on glass in 1857. NPs are usually prepared by physical or chemical methods, and each has its own advantages and problems. Physical methods such as vapour deposition and laser ablation do not offer complete control over the size of the NPs unless at extreme conditions. These typically require a solid substrate, limiting their use in producing liquid suspensions. Chemical methods involve the reduction of the metal ions dissolved in appropriate solvents and for noble metals have produced NPs down to micron level. However, this method does not work well for metals prone to oxidation.
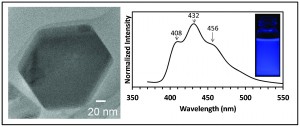
This new paper describes the chemical synthesis of zinc nanoparticles which show exciting photoluminescence properties and a resistance to oxidation. ZnCl was dissolved in a phenylether and complexed with oleylamine before being reduced to Zn2+, after which it nucleates into the final structure. Hexagonal Zn particles form which have a diameter of 250-350nm and showed no oxide phases. The Zn particles also were found to emit light in the UV to blue range. The authors suggest that this is due to the 3d-Fermi gap being lowered compared to bulk zinc, and the sp band itself being discretized.
Find out more:
Chemical synthesis of blue-emitting metallic zinc nano-hexagons
Nguyen T. Mai, Trinh T. Thuy, Derrick M. Mott and Shinya Maenosono
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40801A, Paper
 Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.
Josh Campbell is a PhD student currently at the University of Southampton studying crystal structure prediction of organic semiconductors. He received his BSc from the University of Bradford.