Posted on behalf of Gwenda Kyd, web writer for CrystEngComm
The stability and other properties of compounds in the solid state are important considerations when considering their potential uses. Two strategies commonly followed to enhance desirable properties are to form co-crystals (crystals containing more than one type of neutral molecule) and to form salts (ionic crystals). In both cases, the new crystal forms generated have different properties than the parent compounds. For example, forming a salt of a drug molecule often improves its solubility, hence making it more bioavailable. Salts, however, often have problematic properties including deliquescence (i.e. absorbing water until they dissolve).
A new paper shows how co-crystals can be formed between ionic and neutral forms of the same molecule (conjugate acid-bases, or CABs) which are more stable than the parent molecule. The molecules studied are benzoic acids, salts of which are used as preservatives in the food and pharmaceutical industry. However, they are hygroscopic and degrade in air. Formation of CAB co-crystals significantly improves their stability, particularly at high relative humidity (RH), as shown in the figure below. X-ray crystal structures of the CAB co-crystals show formation of strong short hydrogen bonds between the components. The authors postulate that this interaction is more favourable than the interaction with water, and is the root of the improved stability.
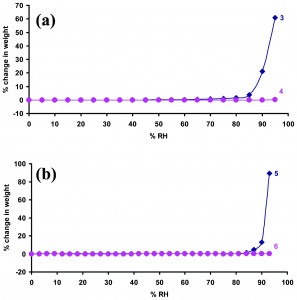
Moisture sorption profiles of (a) sodium benzoate (mono salt), 3, sodium dihydrogen tris(benzoate) (CAB cocrystal), 4, and (b) potassium benzoate (mono salt), 5, and potassium hydrogen bis(benzoate) (CAB cocrystal), 6.
Find out more from the paper:
Improved solid-state stability of salts by cocrystallization between conjugate acid–base pairs
Sathyanarayana Reddy Perumalla and Changquan Calvin Sun
CrystEngComm, 2013, DOI: 10.1039/C3CE40593A











