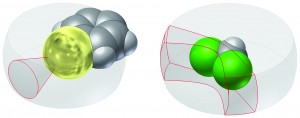
Supramolecular chemistry studies noncovalent bonding such as intermolecular interactions. One of these, the carbon-halogen bond, has generated much interest due to its applications in crystal engineering and drug design. Many statistical studies of chemical structures found in databases such as the Cambridge Structural Database (CSD) have been made in order to gain insight into molecules incorporating this bond.
In this paper, the authors present a new and more accurate method to analyse CSD data for molecules incorporating halogen atoms. This method has a more accurate angular correction, takes into account competitive supramolecular interactions of the halogen atom (e.g. hydrogen bonds), and also allows significant bond directionalities to be found.
Find out more from their paper:
Halogen bonding versus hydrogen bonding: what does the Cambridge Database reveal?
Tiddo J. Mooibroek and Patrick Gamez
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40285A, Paper