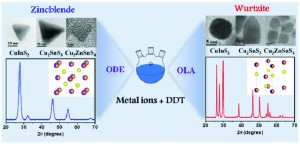
CuInS2 belongs to a class of inorganic semiconductors known as metal chalcogenides, which have desirable optical properties that make them useful in photovoltaics. In solar cell applications, CuInS2 is usually deposited as nanocrystals, and the size, shape and structure of these crystals determine the properties of the device. Therefore, it will be desirable to have a synthetic method that can produce crystals with a uniform morphology, and to be able to vary this by changing the synthetic conditions.
Jin Chang and Eric R. Waclawik have achieved this by a wet-chemical method in which CuInS2 forms nanocrystals with a zincblende structure when a weak-coordinating solvent is used, whereas strong-coordinating solvents produce a wurzite structure. The authors were also able to explain the effect by investigating the intermediate species formed during the chemical reaction. This synthetic method was also extended to two other useful materials: Cu2SnS3 and Cu2ZnSnS4, and they were able to produce pure zincblende or wurzite structures depending on the solvent used.
The synthetic process presented in this paper has the potential to be used in fine tuning the optoelectronic properties of photovoltaic materials, thus yielding better devices for solar energy generation.
Read their article to find out more.
Controlled synthesis of CuInS2, Cu2SnS3 and Cu2ZnSnS4 nano-structures: insight into the universal phase-selectivity mechanism
Jin Chang and Eric R. Waclawik
CrystEngComm, 2013, Advance Article
DOI: 10.1039/C3CE40284C