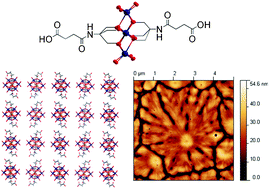
 Noriaki Kubota and colleagues have modelled the effects of sensitivity and resolution of a nucleation detector on metastable zone width.
Noriaki Kubota and colleagues have modelled the effects of sensitivity and resolution of a nucleation detector on metastable zone width.
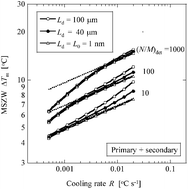
The metastable zone width has been somewhat ambiguously defined as “the supercooling at which the first crystals or uncontrolled spontaneous crystallisation is detected when the solution temperature is lowered at a constant rate”. In order to pin down the specifics, a couple of groups have defined the metastable zone width as the supercooling that occurs when the number density of crystals reaches a fixed value – which in effect is determined by the sensitivity of the detection method.
To find out what effect the sensitivity and resolution of a detector has on metastable zone width readings, the team have employed analytical and numerical methods. A deeper understanding of this is neccesary to enable better control of crystallisation processes.
Download the article today to find out more…
Analytical and numerical study of detector sensitivity and resolution effects on metastable zone width
Noriaki Kubota, Masanori Kobari and Izumi Hirasawa
CrystEngComm, 2013, 15, 2091-2098
DOI: 10.1039/C2CE26968F, Paper