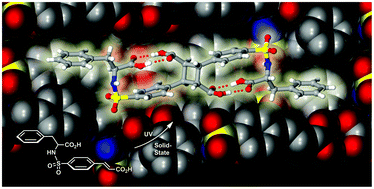
In their 2011, CrystEngComm article, the team reported on the development of a sulfonamidecinnamic acid framework which could be likened to the shape of a fish-hook. Upon crystallisation, the structures form supramolecular dimers where the neighbouring C=C double bonds are oriented mutally co-planar. It is this feature that allows UV initiated dimerisation into the corresponding cyclobutane product.
In their latest CrystEngComm, they extend this study by looking at a greater range of frameworks with different R substituents. To see what impact this has on the supramolecular photodimerisation, download the article now for FREE:
Solid-state photodimerization reactions of racemic and homochiral phenylalanine sulfonamidecinnamic acids
Zhiqing Yan, Andrew J. Bolokowicz, Teage K. Collett, Sarah A. Reeb, Joshua D. Wiseman and Kraig A. Wheeler
CrystEngComm, 2012, Advance Article
DOI: 10.1039/C2CE26307F
Also of interest:
Enantiocontrolled solid-state photodimerizations via a chiral sulfonamidecinnamic acid
Kraig A. Wheeler, Joshua D. Wiseman and Rebecca C. Grove
CrystEngComm, 2011, 13, 3134–3137
DOI: 10.1039/C0CE00516A