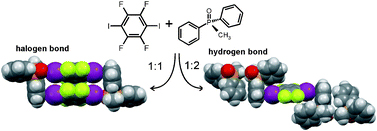
Tomislav Friscic and colleagues report a cocrystal system that bonds by either hydrogen or halogen bonding depending on the stoichiometric ratio of the two components of the crystal. Interactions within crystal structures such as hydrogen bonding, ionic bonds, van der Waals forces and pi-interactions determine a material’s structure and properties.
Understanding these interactions and engineering crystals with specific structures is important, the ability to switch the interaction between hydrogen and halogen bonds by altering the ratio of molecules is an interesting discovery and helps establish the role halogen bonds play in molecular self-assembly.
Switching between halogen- and hydrogen-bonding in stoichiometric variations of a cocrystal of a phosphine oxide
Se Ye Oh, Christopher W. Nickels, Felipe Garcia, William Jones and Tomislav Friščić