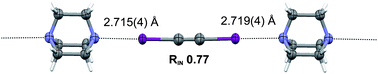Lee Brammer and colleagues report crystal structures of halogen-bonded adducts between diiodoacetylene and nitrogen/oxygen acceptor groups showing the suitability of diiodoacetylene as a ditopic connector.
The strength in the halogen bond is due to the electron-withdrawing effect from the sp-hybridised carbon atom in diiodoacetyle. Being able to form such strong intermolecular interactions via such a small linear molecule with two conformationally constrained faces makes diiodoacetylene a useful building block in crystal engineering and supramolecular chemistry.
Read the paper for free…
Diiodoacetylene: compact, strong ditopic halogen bond donor
Catherine Perkins, Stefano Libri, Harry Adams and Lee Brammer
CrystEngComm, 2012
DOI: 10.1039/C2CE00029F
Here is a selection of other recent work from Lee Brammer and colleagues…
Different structural destinations: comparing reactions of [CuBr2(3-Brpy)2] crystals with HBr and HCl gas
Guillermo Mínguez Espallargas, Alastair J. Florence, Jacco van de Streek and Lee Brammer
CrystEngComm, 2011, 13, 4400-4404
DOI: 10.1039/C1CE05222E
From themed issue Dynamic behaviour and reactivity in crystalline solids
Synthesis and polymorphism of (4-ClpyH)2[CuCl4]: solid–gas and solid–solid reactions
Iñigo J. Vitorica-Yrezabal, Rachel A. Sullivan, Stephen L. Purver, Caroline Curfs, Chiu C. Tang and Lee Brammer
CrystEngComm, 2011, 13, 3189-3196
DOI: 10.1039/C0CE00628A
From themed issue Reactions in molecular solids and host–guest systems
Effects of halogen bonding in ferromagnetic chains based on Co(II) coordination polymers
Juan M. Clemente-Juan, Eugenio Coronado, Guillermo Mínguez Espallargas, Harry Adams and Lee Brammer
CrystEngComm, 2010, 12, 2339-2342
DOI: 10.1039/C003078C
From themed issue New Talent