 Professor Gautam Desiraju was born in Madras, India, and received his B.Sc. at the University of Bombay, India, in 1972. He was awarded his Ph.D. from the University of Illinois at Urbana-Champaign, USA, in 1976. After two years at Eastman Kodak Company, Rochester, New York, USA, he joined the faculty at the University of Hyderabad, India. He left the University of Hyderabad in 2009 and is currently at the Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. He was recently was elected President of the International Union of Crystallography (IUCr) during the IUCr General Assembly in Madrid, Spain for the triennium 2011-2014.
Professor Gautam Desiraju was born in Madras, India, and received his B.Sc. at the University of Bombay, India, in 1972. He was awarded his Ph.D. from the University of Illinois at Urbana-Champaign, USA, in 1976. After two years at Eastman Kodak Company, Rochester, New York, USA, he joined the faculty at the University of Hyderabad, India. He left the University of Hyderabad in 2009 and is currently at the Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore. He was recently was elected President of the International Union of Crystallography (IUCr) during the IUCr General Assembly in Madrid, Spain for the triennium 2011-2014.
Prof. Desiraju was one of the founder members of the CrystEngComm Editorial Board and has also served on the Editorial Advisory Board of ChemComm. His 1989 book on crystal engineering and 1995 review in Angewandte Chemie on supramolecular synthons redefined several aspects of the subject of crystal engineering, and in particular led to an emphasis on the study of hydrogen bonds and other intermolecular interactions.
Why did you want to become a scientist?
I always liked chemistry, I was fascinated by it. During my first lab experiment in chemistry I remember thinking I didn’t want to do anything else. I was very lucky that I had the opportunity to do what I liked.
What projects are you working on at the moment?
So many projects that I have lost count! Currently I have at least 10-15 projects underway in my group. We are very excited about nanoindentation, which is a new technique that allows us to experimentally compare the interaction strengths and monitor anisotropy of molecular crystals. This requires a very good student as it is a very laborious process – you need to know the faces of the crystal really well.
What do you think will be the next big breakthrough in your field?
If I knew what it was, I would be doing it! The beauty of scientific research is that you never know. If you could predict the next breakthrough, everyone would have gotten there!
How do you think Crystal Engineering will develop in the next couple of years?
There is no doubt that crystal engineering has spread far and wide. Unlike other areas in crystallography, it has attracted lots of people and interest, even though it is hard. Big ideas in chemistry are sustained when there is commercial application because this brings in money to attract research. Crystal engineering is very lucky as areas like metal-organic frameworks and pharmaceuticals are very big and have lots of money, and so there is lots of interest. The commercial applications and the challenge are an irresistible temptation. No subject addressed by the both the RSC and ACS can be small.
According to the evolutionary model it is the survival of the fittest, and so the not so good areas of science will die out because of the lack of support. These are not good times for science, and we do need to worry about it. However, the future is very bright for crystal engineering, as the pharmaceutical industry sustains the organic research, and the chemical industry sustains the metal-organic framework research.
What is the most rewarding aspect of your work?
Just the fun of doing it! Scientists like me are very lucky as we work with young people all the time, which keeps both the scientist and the young person active. Other careers such as medicine, police, etc. are not so happy because they are not exposed to young people as much, but we are fortunate enough to see the ‘innocence of life’ all the time.
What is the secret to a successful research group?
This really depends on the personality of the research advisor, because they are the central person in the group. As the manager it is their role to get the best output possible out of the team depending on how they motivate the people around them. It is a very fine balance between happiness and productivity. There should be an abundance of both in every research group.
What achievement are you most proud of?
Pride is a bad word. All scientists like to do their own thing. Serious scientists would do this irrespective of anything. It is best when you do it for yourself and it interests your peers. For my plenary at the IUCr Congress in Madrid I specifically kept the material to be recent, and even though there was a large lecture hall full of people at 9am on the last day of the conference, I could have easily have given that talk to myself, as it gives me the greatest happiness to explore my work.
What advice would you give to a young scientist?
Go have fun in the lab. Be bold and do what you like to. Let go of inhibition and remember that your training is only a platform so you don’t do nonsense. Research is highly individual, and you have to do your own thing. Think like this from the very beginning.
What would you do if you weren’t a scientist?
I would have studied history or sociology.
What is your favourite space group and why?
P21/c with Z’=1 (not 0.5 + 0.5), because this is the norm, and merits no further consideration. Anything else needs a chemical explanation, as this condition requires no chemistry. What is not P21/c with Z’=1 is crystal engineering/me.
What was your first crystal structure?
It was horrible! I did my PhD in the US in 1975, and we had to hand-centre the reflections before data collection! It was hard work and lots of effort to turn arcs on this enormous machine. The structure was in Pbca with a c-axis of 44Å and very close spots. I was baptised by fire! You never forget the first!
Find out more about Gautam Desiraju on his webpage at the Solid State and Structural Chemistry Unit, Indian Institute of Science, Bangalore
Read some of Gautam’s exciting research in the following articles:
Shape and size mimicry in the design of ternary molecular solids: towards a robust strategy for crystal engineering
S. Tothadi, A. Mukherjee and Gautam R. Desiraju
Chem. Commun., 2011, Advance Article
DOI: 10.1039/C1CC14567C
Drug-drug co-crystals: Temperature-dependent proton mobility in the molecular complex of isoniazid with 4-aminosalicylic acid
Pawel Grobelny, Arijit Mukherjee and Gautam R. Desiraju
CrystEngComm, 2011, 13, 4358-4364
From themed issue Dynamic behaviour and reactivity in crystalline solids
Nature and strength of C–H···O interactions involving formyl hydrogen atoms: computational and experimental studies of small aldehydes
Tejender S. Thakur, Michael T. Kirchner, Dieter Bläser, Roland Boese and Gautam R. Desiraju
Phys. Chem. Chem. Phys., 2011, 13, 14076-14091
From themed issue Weak hydrogen bonds – strong effects?
 Copper sulfide cages wholly exposed with nanotwinned building blocks
Copper sulfide cages wholly exposed with nanotwinned building blocks 












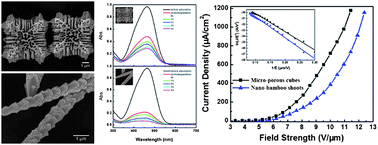 In this CrystEngComm HOT Article, two kinds of micro/nano-sized single-crystalline cuprous oxide (Cu2O) crystals with novel porous cubic or hierarchical rod-like morphologies were successfully synthesized via a facile ethanol-assisted double-solvothermal method. It was found that the addition of ethanol in precursor solution is critical for the formation of hierarchical rod-like structures (nano-bamboo shoots). Their growth process and shape evolution together with their optical properties and field emission have also been reported.
In this CrystEngComm HOT Article, two kinds of micro/nano-sized single-crystalline cuprous oxide (Cu2O) crystals with novel porous cubic or hierarchical rod-like morphologies were successfully synthesized via a facile ethanol-assisted double-solvothermal method. It was found that the addition of ethanol in precursor solution is critical for the formation of hierarchical rod-like structures (nano-bamboo shoots). Their growth process and shape evolution together with their optical properties and field emission have also been reported.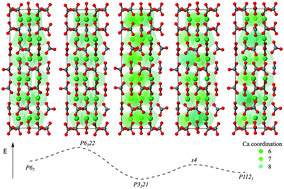 Vaterite is one of the three crystalline polymorphs of calcium carbonate, and plays a significant role in biomineralisation, either as an intermediate formed from amorphous calcium carbonate prior to transformation to aragonite or calcite, or can be utilized by organisms in its own right.
Vaterite is one of the three crystalline polymorphs of calcium carbonate, and plays a significant role in biomineralisation, either as an intermediate formed from amorphous calcium carbonate prior to transformation to aragonite or calcite, or can be utilized by organisms in its own right.
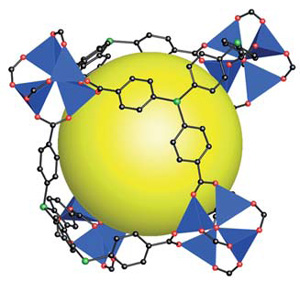


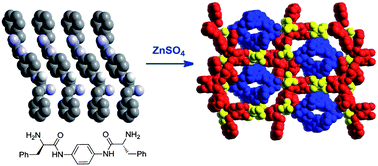 In this HOT Article, two new phenyl-bridged pseudopeptidic ligands have been prepared and structurally characterised. The nature of the ligands’ substituents play an important role in the nature of the solid state structure yielding either hydrogen bonded linked sheets of molecules or infinite hydrogen bonded networks. To investigate this further, these ligands were reacted with a range of zinc(II) salts with the aim of synthesising coordination polymers and networks; the role of anions in determining the final structure was explored.
In this HOT Article, two new phenyl-bridged pseudopeptidic ligands have been prepared and structurally characterised. The nature of the ligands’ substituents play an important role in the nature of the solid state structure yielding either hydrogen bonded linked sheets of molecules or infinite hydrogen bonded networks. To investigate this further, these ligands were reacted with a range of zinc(II) salts with the aim of synthesising coordination polymers and networks; the role of anions in determining the final structure was explored.