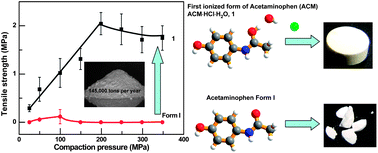
 This HOT article reports the first salt form of acetaminophen, which is shown to have superior tableting properties relative to the stable polymorph of the unionised drug. Amide groups are generally considered non-ionisable for the purposes of drug development and there are only a handful of crystal structures with ionised amide groups known. This success of forming an amide–hydrochloride salt using a simple experimental protocol may well encourage other research groups, both industrial and academic, to attempt salt formation with amides.
This HOT article reports the first salt form of acetaminophen, which is shown to have superior tableting properties relative to the stable polymorph of the unionised drug. Amide groups are generally considered non-ionisable for the purposes of drug development and there are only a handful of crystal structures with ionised amide groups known. This success of forming an amide–hydrochloride salt using a simple experimental protocol may well encourage other research groups, both industrial and academic, to attempt salt formation with amides.
Read more about this important contribution to solid-state chemistry for FREE until 10th January 2012 at:
Ionized form of acetaminophen with improved compaction properties
Sathyanarayana Reddy Perumalla, Limin Shi and Changquan Calvin Sun
CrystEngComm, 2012, Advance Article
DOI: 10.1039/C1CE06278F











