 In this CrystEngComm Hot article, Roberto Otero and colleagues from Spain self assemble zinc meso-tetramesitylporphyrin on copper and gold into one-dimensional nanorods.
In this CrystEngComm Hot article, Roberto Otero and colleagues from Spain self assemble zinc meso-tetramesitylporphyrin on copper and gold into one-dimensional nanorods.
Otero explains they are ‘shish-kebab type coordination polymers’, and that they can extend for hundreds of nanometres.
Read the full article for FREE to find out more about these porphyrin nanorods…
Surface assembly of porphyrin nanorods with one-dimensional zinc–oxygen spinal cords
Marta Trelka, Christian Urban, Celia Rogero, Paula de Mendoza, Eva Mateo-Marti, Yang Wang, Iñaki Silanes, David Écija, Manuel Alcamí, Felix Yndurain, Andrés Arnau, Fernando Martín, Antonio M. Echavarren, José Ángel Martín-Gago, José María Gallego, Roberto Otero and Rodolfo Miranda
CrystEngComm, 2011, DOI: 10.1039/C1CE05494E
Keep up to date with the latest news and research in solid-state and crystalline materials: sign up to the CrystEngComm e-alert, follow us on Twitter, and get the RSS feed.











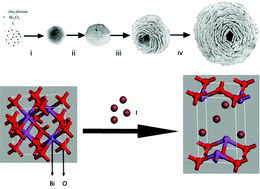 Read this CrystEngComm Hot article to discover how the Kirkendall effect can be used to make BiOI nests.
Read this CrystEngComm Hot article to discover how the Kirkendall effect can be used to make BiOI nests.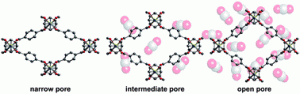
 In this HOT article, Babashkina and co-workers have synthesized the first examples of NiII complexes containing the same asymmetric NTT [(N-(thio)phosphorylated thioamides and thioureas RC(S)NHP(X)(OR0)2 (X ¼ O, S)] ligand featuring an aryl-NH substituent at the thiocarbonyl group and coordinating to the metal both in the 1,3-N,S- and 1,5-S,S’-fashion in the solid state depending on the crystallization conditions.
In this HOT article, Babashkina and co-workers have synthesized the first examples of NiII complexes containing the same asymmetric NTT [(N-(thio)phosphorylated thioamides and thioureas RC(S)NHP(X)(OR0)2 (X ¼ O, S)] ligand featuring an aryl-NH substituent at the thiocarbonyl group and coordinating to the metal both in the 1,3-N,S- and 1,5-S,S’-fashion in the solid state depending on the crystallization conditions. Song Gao and colleagues from Peking University investigate a way of improving the chances of obtaining chiral crystalline solids, in this CrystEngComm Hot article.
Song Gao and colleagues from Peking University investigate a way of improving the chances of obtaining chiral crystalline solids, in this CrystEngComm Hot article.
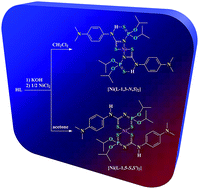
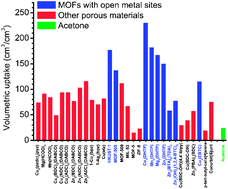 Acetylene is an important starting material in the petrochemical and electronic industry for various industrial and consumer products, and a promising alternative energy source for future acetylene fuel cell vehicles. With the importance of acetylene, as a green fuel, this CrystEngComm Highlight, focuses on the storage of acetylene and its separation from the CO2, CH4, or C2H4 mixtures on micro-porous metal–organic frameworks (MOFs).
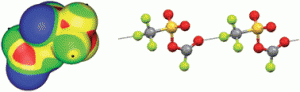
Acetylene is an important starting material in the petrochemical and electronic industry for various industrial and consumer products, and a promising alternative energy source for future acetylene fuel cell vehicles. With the importance of acetylene, as a green fuel, this CrystEngComm Highlight, focuses on the storage of acetylene and its separation from the CO2, CH4, or C2H4 mixtures on micro-porous metal–organic frameworks (MOFs).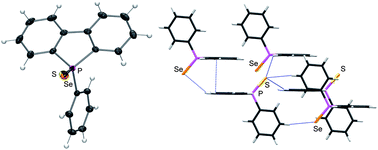 In this HOT article, the solid-state interactions of dibenzophosphole chalcogenides and their potential application as electron transporting materials were investigated. The crystallographic properties of 9-phenyl-9-dibenzophosphole chalcogenides were compared with the mixed crystal structure and optical properties of 9-phenyl-9-dibenzophosphole sulphideselenide. The mixed crystal displayed desirable properties, i.e. carrier transport and emission properties, and this approach may be useful in the future for optoelectronic applications such as light-emitting SC-OFETs
In this HOT article, the solid-state interactions of dibenzophosphole chalcogenides and their potential application as electron transporting materials were investigated. The crystallographic properties of 9-phenyl-9-dibenzophosphole chalcogenides were compared with the mixed crystal structure and optical properties of 9-phenyl-9-dibenzophosphole sulphideselenide. The mixed crystal displayed desirable properties, i.e. carrier transport and emission properties, and this approach may be useful in the future for optoelectronic applications such as light-emitting SC-OFETs