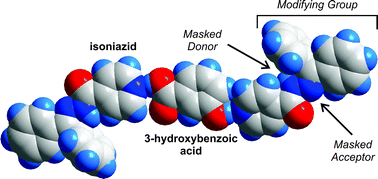
 How does one control and modify the self-assembly process of organic molecules towards a desired solid state? Andreas Lemmerer, Joel Bernstein and Volker Kahlenberg ask themselves this very question in their recent CrystEngComm Hot Article. In their paper the group tell us how they modify the hydrogen bonding in isonicotinic acid hydrazide (isoniazid) in order to control the self-assembly process.
How does one control and modify the self-assembly process of organic molecules towards a desired solid state? Andreas Lemmerer, Joel Bernstein and Volker Kahlenberg ask themselves this very question in their recent CrystEngComm Hot Article. In their paper the group tell us how they modify the hydrogen bonding in isonicotinic acid hydrazide (isoniazid) in order to control the self-assembly process.
Isoniazid is an active pharmaceutical ingredient that helped cure tuberculosis as part of a triple therapy cocktail. It co-crystallizes with carboxylic acids to form pharmaceutical co-crystals and is also a versatile supramolecular reagent as it has multiple donor and accepting groups to interact with different functional groups. Find out more about this study and Isoniazid in the article – FREE to read until 31 May 2011.
Covalent assistance in supramolecular synthesis: in situ modification and masking of the hydrogen bonding functionality of the supramolecular reagent isoniazid in co-crystals
Andreas Lemmerer, Joel Bernstein and Volker Kahlenberg
CrystEngComm, 2011, Advance Article DOI: 10.1039/C1CE05152K, Paper










