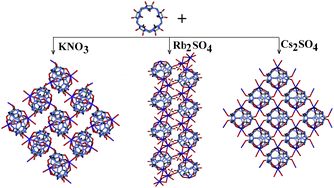
Designing metal–organic frameworks (MOFs) with structure-based properties has received a considerable amount of interest in recent years. This is due to their fascinating molecular structures and potential applications in molecular adsorption, separation, heterogeneous catalysis, magnetism and electrical conductivity.
The basic strategy for constructing MOFs is to use an appropriate organic ligand, especially bridging ligands containing multi-dentate oxygen or nitrogen donors, to coordinate to the metal centers. Cucurbit[n]uril (n = 5–8, 10, abbreviated as Q[n]) is a class of barrel-shaped organic macrocyclic ligand with identical carbonyl-laced portals on each side. The polar carbonyl groups of the Q[n]s are capable of metal-coordination and therefore Q[n]s are promising building blocks for the construction of coordination polymers.
Now in this CrystEngComm Hot Article, Zhu Tao et al., report the synthesis and crystal structures of three coordination polymers with K+, Rb+ and Cs+ coupled with (HO)10Q[5] as the building block.
Read this article for free until 5th May 2011 here.
Coordination polymers constructed from alkali metal ions and (HO)10cucurbit[5]uril
Xin Xiao, Zhu Tao, Sai-Feng Xue, Yun-Qian Zhang, Qian-Jiang Zhu, Jing-Xin Liu and Gang Wei
CrystEngComm, 2011, Advance Article, DOI: 10.1039/C1CE05162H