A faster, more informative way to probe protein structures, which are vital to understanding protein function, has been devised by researchers in the US.
Proteins drive most biological processes but the majority of studies on them are performed under non-biological conditions where key dynamic effects can’t be captured. For example, crystallography uses solid state, and mass spectrometry is in the gas phase and can be denaturing.
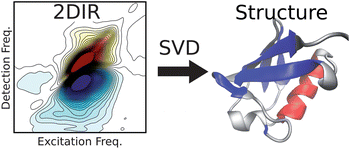
This method, developed by Carlos Baiz from the Andrei Tokmakoff group at Massachusetts Institute of Technology, uses two-dimensional infrared spectroscopy, and offers ultrafast time resolution, and enables study in solution, which provides the potential to look at protein folding/unfolding pathways; this could considerably expand scientists’ understanding of the protein structure/function relationship.
Coherent two-dimensional infrared spectroscopy: Quantitative analysis of protein secondary structure in solution
Carlos R. Baiz, Chunte Sam Peng, Mike E. Reppert, Kevin C. Jones and Andrei Tokmakoff
Analyst, 2012, Advance Article
DOI: 10.1039/C2AN16031E