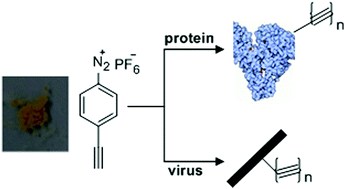
A recent communication in Organic and Biomolecular Chemistry reports the synthesis of a new reagent for the introduction of alkynes into proteins; a key step for click bioconjugation. The new diazonium reagent was prepared in a one-pot synthesis from commercially available 4-ethynylaniline.
Having optimized bioconjugation in a tyrosine containing small molecule, the new diazonium reagent was then used to covalently label proteins. Fluorescence labelling of protein surface tyrosine residues was achieved under mild conditions (pH 8.0) by reaction of the protein with the diazonium reagent and subsequent reaction with an azide-containing fluorescent compound. The new diazonium reagent was also used to achieve protein PEGylation; a strategy that could be employed to improve protein stability and reduce immunogenicity.
The new diazonium reagent can facilitate bioconjugation in a range of proteins and could be a useful addition to the biochemist’s toolbox.
Read the full article:
An efficient reagent for covalent introduction of alkynes into proteins
Jie Zhang, Dejun Ma, Dawei Du, Zhen Xi and Long Yi
Org. Biomol. Chem., 2014, DOI: 10.1039/C4OB01873G











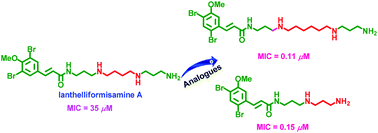 There is a need to develop new antibiotics to combat the emergence of antibiotic resistant bacterial pathogens. In bacteria, polyamine analogues can compete with natural polyamines to affect key cellular processes. Taking inspiration from the antibacterial properties of bromotyrosine-derived alkaloids isolated from the marine sponge (Subarea ianthelliformis), a
There is a need to develop new antibiotics to combat the emergence of antibiotic resistant bacterial pathogens. In bacteria, polyamine analogues can compete with natural polyamines to affect key cellular processes. Taking inspiration from the antibacterial properties of bromotyrosine-derived alkaloids isolated from the marine sponge (Subarea ianthelliformis), a