 The cyclobutane ring is a unique structural element found in a wide variety of biologically active natural products and synthetic molecules. Although cyclobutanes have been known for centuries, as a result of inherent ring strain, their application in synthesis has only become more popular in the last 40-50 years.
The cyclobutane ring is a unique structural element found in a wide variety of biologically active natural products and synthetic molecules. Although cyclobutanes have been known for centuries, as a result of inherent ring strain, their application in synthesis has only become more popular in the last 40-50 years.
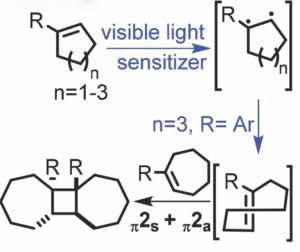
The photochemical [2+2] cycloaddition of alkenes represents a powerful strategy for the synthesis of cyclobutane rings. However, direct irradiation of cycloalkenes with UV light often leads to unwanted and difficult to control rearrangement pathways.
Professor Jimmie Weaver of Oklahoma State University proposes an alternative to direct irradiation of cycloalkenes by instead capturing energy in the form of ring strain. The Weaver group has applied their mild and efficient methodology toward the synthesis of cyclobutane rings imbedded within a C2-symmetric tricyclic framework.
It is well known that thermal [2+2] cycloadditions are ‘forbidden’ processes due to unfavourable orbital overlap of the reaction partners during the transition state. However, a common exception to this is the [π2s+π2a] addition of alkenes and ketenes. The Weaver group proposes that a thermal [π2s+π2a] cycloaddition could take place for ground state alkenes by generating a high energy intermediate, which would result in a decreased relative energy barrier for the thermal cycloaddition.
This method uses an iridium-based photocatalyst to generate the highly strained trans-cycloheptene intermediate—which possesses 27-36 kcal/mol of ring strain—in order to drive the thermal [2+2] cycloaddition of cycloheptenes and various cycloalkene substrates. Interestingly, the reaction results in four new stereocenters which are generated with excellent stereoselectivity and regioselectivity. An added advantage of using light within the visible spectrum to activate the photocatalyst minimizes competitive photochemical [2+2] addition pathways.
This study is an excellent example of the application of basic principles to drive previously inaccessible mechanistic pathways. The authors hope that their study will encourage other applications of visible light energy to drive unfavourable endergonic reactions.
To find out more see:
An elusive thermal [2+2] cycloaddition driven by visible light photocatalysis: tapping into strain to access C2-symmetric tricyclic rings
Kamaljeet Singh,
Victoria Corless has recently completed her Ph.D. in organic chemistry with Prof. Andrei Yudin at the University of Toronto. Her research is centred on the synthesis of kinetically amphoteric building blocks which offer a versatile platform for the development of chemoselective transformations with particular emphasis on creating novel biologically active molecules. She is passionate about communicating new discoveries to enhance science literacy.










