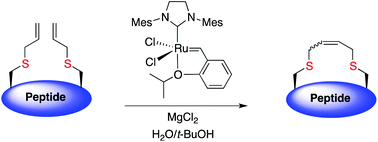
Disulfide bridges are critical for peptide folding, stability and biological activity. This technique enables the replacement of a disulfide bridge with a dicarba linkage, improving stability yet retaining biological activity.
The trick to performing these cyclisations in the aqueous phase is to use tert-butanol or detergent micelles to solubilise the ruthenium catalyst. The addition of magnesium chloride is required to disrupt non-productive chelation between the ruthenium-carbene and unprotected hydroxyl, carboxyl and primary amide functionalities. Low yields with non-sulphur containing peptide analogues indicate that the sulphur atom is a prerequisite for successful ring-closing metathesis in these peptides.
This technique opens up the possibility of performing cyclisations that cannot be achieved on-resin, such as the ring-closing metathesis of crotalphine analogues (due to either complex formation between catalyst and amide backbone and/or hydrophobic peptide protecting groups). Additionally, this method avoids extensive synthetic effort to modify the ruthenium catalyst to optimise aqueous solubility.
Investigation of the ring-closing metathesis of peptides in water
Stephen A. Cochrane, Zedu Huang and John C. Vederas
DOI: 10.1039/C2OB26938D
Published on behalf of Steve Moore, Organic & Biomolecular Chemistry web science writer.










