Posted on behalf of Liana Allen, web writer for Dalton Transactions
Chemotherapy remains the most widely used therapeutic approach to treating cancer. Such drugs work by targeting and killing rapidly dividing cells (a major characteristic of most cancer cells) by impairing mitosis (cell division).1 These drugs are often highly effective at suppressing or even eliminating cancer in the patient, however, they can also lead to many severe side effects such as immunosuppression and infertility. Free radicals and reactive oxygen species (ROS), a by-product of the chemotherapy process, are frequently implicated as a cause of some of the side effects experienced.2 Hence, a drug which simultaneously kills cancer cells and scavenges free radicals and ROS could hypothetically reduce the side effects of chemotherapy while remaining effective against the disease.
In this article, the authors combine two biologically active species into discrete potential drug molecules; hydroxyl-substituted Schiff bases, known to have good free radical scavenging and anti-cancer activity,3 and ferrocene, previously shown to increase the anti-cancer activity of other chemotherapeutic drugs.4
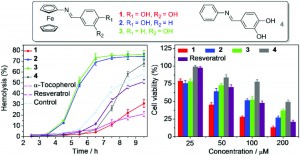
Using established methods, the authors thoroughly tested their new compounds for anti-free radical and anti-cancer activity. The results showed that one molecule in particular (“Compound 1”) has good free radical scavenging activity against ABTS.- and DPPH. (assessed by changes in UV-Vis absorbance), as well as displaying cytoprotective activity against radical attacks, delaying free radical oxidative damage to membrane cells. “Compound 1” was also shown to possess good anti-cancer activity against HeLa cancer cell lines, even out-performing clinically used anti-cancer drug Resveratrol. These early findings show promise for the development of chemotherapy treatments which combine antioxidant and anti-cancer activities.
To read more, see:
Synthesis and biological evaluation of hydroxyl-substituted Schiff-bases containing ferrocenyl moieties
Wansong Chen, Weizhu Ou, Liqiang Wang, Yuanqiang Hao, Jianshun Cheng, Juan Li and You-Nian Liu,
Dalton Trans., 2013, DOI: 10.1039/C3DT51977E, Paper
1 V. Malhorta, M. C. Perry, Cancer Biol. Ther., 2003, 2 (Suppl. 1), S2-4.
2 E.-S. E. El-Awady, Y. M. Moustfa, D. M. Abo-Elmatty and A. Radwan, Eur. J. Pharmacol., 2011, 650, 335-341.
3 Y.-F. Li and Z.-Q. Liu, Eur. J. Pharm. Sci., 2011, 44, 158-163.
4 B. Zhou, J. Li, B.-J. Feng, Y. Ouyang, Y.-N. Liu and F. Zhou, J. Inorg. Biochem., 2012, 116, 19-25.
 Dr. C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.
Dr. C. Liana Allen is currently a post-doctoral research associate in the group of Professor Scott Miller at Yale University, where she works on controlling the enantio- or regioselectivity of reactions using small peptide catalysts. Liana received her Ph.D. in organic chemistry at Bath University with Professor Jonathan Williams, where she worked on developing novel, efficient syntheses of amide bonds.